Trypanosoma cruzi expresses a plant-like ascorbate-dependent hemoperoxidase localized to the endoplasmic reticulum
- PMID: 12351682
- PMCID: PMC129694
- DOI: 10.1073/pnas.202422899
Trypanosoma cruzi expresses a plant-like ascorbate-dependent hemoperoxidase localized to the endoplasmic reticulum
Abstract
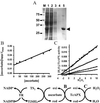
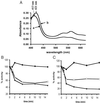
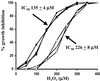
In most aerobic organisms hemoperoxidases play a major role in H(2)O(2)-detoxification, but trypanosomatids have been reported to lack this activity. Here we describe the properties of an ascorbate-dependent hemoperoxidase (TcAPX) from the American trypanosome Trypanosoma cruzi. The activity of this plant-like enzyme can be linked to the reduction of the parasite-specific thiol trypanothione by ascorbate in a process that involves nonenzymatic interaction. The role of heme in peroxidase activity was demonstrated by spectral and inhibition studies. Ascorbate could saturate TcAPX activity indicating that the enzyme obeys Michaelis-Menten kinetics. Parasites that overexpressed TcAPX activity were found to have increased resistance to exogenous H(2)O(2). To determine subcellular location an epitope-tagged form of TcAPX was expressed in T. cruzi, which was observed to colocalize with endoplasmic reticulum resident chaperone protein BiP. These findings identify an arm of the oxidative defense system of this medically important parasite. The absence of this redox pathway in the human host may be therapeutically exploitable.
Figures


Similar articles
-
The Trypanosoma cruzi vitamin C dependent peroxidase confers protection against oxidative stress but is not a determinant of virulence.PLoS Negl Trop Dis. 2015 Apr 13;9(4):e0003707. doi: 10.1371/journal.pntd.0003707. eCollection 2015 Apr. PLoS Negl Trop Dis. 2015. PMID: 25875298 Free PMC article.
-
Crystal structure of Trypanosoma cruzi heme peroxidase and characterization of its substrate specificity and compound I intermediate.J Biol Chem. 2022 Aug;298(8):102204. doi: 10.1016/j.jbc.2022.102204. Epub 2022 Jun 27. J Biol Chem. 2022. PMID: 35772495 Free PMC article.
-
The level of ascorbate peroxidase is enhanced in benznidazole-resistant populations of Trypanosoma cruzi and its expression is modulated by stress generated by hydrogen peroxide.Mem Inst Oswaldo Cruz. 2012 Jun;107(4):494-502. doi: 10.1590/s0074-02762012000400009. Mem Inst Oswaldo Cruz. 2012. PMID: 22666860
-
Peroxidases of trypanosomatids.Antioxid Redox Signal. 2008 Sep;10(9):1593-606. doi: 10.1089/ars.2008.2050. Antioxid Redox Signal. 2008. PMID: 18498224 Review.
-
The role of glutathione peroxidases in trypanosomatids.Biol Chem. 2003 Apr;384(4):517-25. doi: 10.1515/BC.2003.060. Biol Chem. 2003. PMID: 12751782 Review.
Cited by
-
Reduction of anti-leishmanial pentavalent antimonial drugs by a parasite-specific thiol-dependent reductase, TDR1.Biochem J. 2004 Jul 15;381(Pt 2):405-12. doi: 10.1042/BJ20040283. Biochem J. 2004. PMID: 15056070 Free PMC article.
-
Ascorbate peroxidase, a key molecule regulating amphotericin B resistance in clinical isolates of Leishmania donovani.Antimicrob Agents Chemother. 2014 Oct;58(10):6172-84. doi: 10.1128/AAC.02834-14. Epub 2014 Aug 11. Antimicrob Agents Chemother. 2014. PMID: 25114128 Free PMC article.
-
Evasion of the Immune Response by Trypanosoma cruzi during Acute Infection.Front Immunol. 2016 Jan 18;6:659. doi: 10.3389/fimmu.2015.00659. eCollection 2015. Front Immunol. 2016. PMID: 26834737 Free PMC article. Review.
-
Evolution of energy metabolism and its compartmentation in Kinetoplastida.Kinetoplastid Biol Dis. 2003 Oct 28;2(1):11. doi: 10.1186/1475-9292-2-11. Kinetoplastid Biol Dis. 2003. PMID: 14613499 Free PMC article.
-
Redox Balance Keepers and Possible Cell Functions Managed by Redox Homeostasis in Trypanosoma cruzi.Front Cell Infect Microbiol. 2019 Dec 20;9:435. doi: 10.3389/fcimb.2019.00435. eCollection 2019. Front Cell Infect Microbiol. 2019. PMID: 31921709 Free PMC article. Review.
References
-
- World Health Organization. Fourteenth Programme Report of the United Nations Development Program/World Bank/World Health Organization, Research and Training in Tropical Diseases (TDR) Geneva: W.H.O.; 1997.
-
- Carnieri E V S, Moreno S N J, Docampo R. Mol Biochem Parasitol. 1993;61:79–86. - PubMed
-
- Fairlamb A H, Cerami A. Annu Rev Microbiol. 1992;46:695–729. - PubMed
-
- Flohe L, Hecht H J, Steinert P. Free Radical Biol Med. 1999;27:966–984. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

