Repressor element-1 silencing transcription/neuron-restrictive silencer factor is required for neural sodium channel expression during development of Xenopus
- PMID: 12351707
- PMCID: PMC6757790
- DOI: 10.1523/JNEUROSCI.22-19-08347.2002
Repressor element-1 silencing transcription/neuron-restrictive silencer factor is required for neural sodium channel expression during development of Xenopus
Abstract
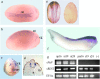
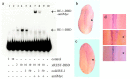
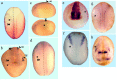
The ability of neurons to fire rapid action potential relies on the expression of voltage-gated sodium channels; the onset of the transcription of genes that encode these channels occurs during early neuronal development. The factors that direct and regulate the specific expression of ion channels are not well understood. Repressor element-1 silencing transcription/neuron-restrictive silencer factor (REST/NRSF) is a transcriptional regulator characterized as a repressor of the expression of NaV1.2, the gene encoding the voltage-gated sodium channel most abundantly expressed in the CNS, as well as of the expression of numerous other neuronal genes. In mammals, REST/NRSF is expressed mostly in non-neural cell types and immature neurons, and it is downregulated on neural maturation. To understand the mechanisms that govern sodium channel gene transcription and to explore the role of REST/NRSF in vivo, we inhibited REST/NRSF action in developing Xenopus laevis embryos by means of a dominant negative protein or antisense oligonucleotides. Contrary to what was expected, these maneuvers result in the decrease of the expression of the NaV1.2 gene, as well as of other neuronal genes in the primary spinal neurons and cranial ganglia, without overt perturbation of neurogenesis. These results, together with the demonstration of robust REST/NRSF expression in primary spinal neurons, suggest that REST/NRSF is required for the acquisition of the differentiated functional neuronal phenotype during early development. Furthermore, they suggest that REST/NRSF may be used to activate or repress transcription of neuronal genes in distinct cellular and developmental contexts.
Figures
Similar articles
-
Brain REST/NRSF Is Not Only a Silent Repressor but Also an Active Protector.Mol Neurobiol. 2017 Jan;54(1):541-550. doi: 10.1007/s12035-015-9658-4. Epub 2016 Jan 7. Mol Neurobiol. 2017. PMID: 26742529 Review.
-
RE-1 silencer of transcription/neural restrictive silencer factor modulates ectodermal patterning during Xenopus development.J Neurosci. 2006 Mar 8;26(10):2820-9. doi: 10.1523/JNEUROSCI.5037-05.2006. J Neurosci. 2006. PMID: 16525062 Free PMC article.
-
Developmental regulation of the expression of sodium currents in Xenopus primary neurons.Biol Res. 2006;39(3):483-91. doi: 10.4067/s0716-97602006000300010. Epub 2006 Nov 7. Biol Res. 2006. PMID: 17106580
-
Regulation of cholinergic gene expression by the neuron restrictive silencer factor/repressor element-1 silencing transcription factor.Life Sci. 2003 Mar 28;72(18-19):2021-8. doi: 10.1016/s0024-3205(03)00065-1. Life Sci. 2003. PMID: 12628452
-
Regulation of the cholinergic gene locus by the repressor element-1 silencing transcription factor/neuron restrictive silencer factor (REST/NRSF).Life Sci. 2004 Mar 19;74(18):2213-25. doi: 10.1016/j.lfs.2003.08.045. Life Sci. 2004. PMID: 15017977 Review.
Cited by
-
Repressor element 1-silencing transcription factor deficiency yields profound hearing loss through Kv7.4 channel upsurge in auditory neurons and hair cells.Elife. 2022 Sep 20;11:e76754. doi: 10.7554/eLife.76754. Elife. 2022. PMID: 36125121 Free PMC article.
-
More than a Corepressor: The Role of CoREST Proteins in Neurodevelopment.eNeuro. 2020 Mar 10;7(2):ENEURO.0337-19.2020. doi: 10.1523/ENEURO.0337-19.2020. Print 2020 Mar/Apr. eNeuro. 2020. PMID: 32075869 Free PMC article. Review.
-
A conserved role but different partners for the transcriptional corepressor CoREST in fly and mammalian nervous system formation.J Neurosci. 2004 Aug 11;24(32):7186-93. doi: 10.1523/JNEUROSCI.0238-04.2004. J Neurosci. 2004. PMID: 15306652 Free PMC article.
-
Brain REST/NRSF Is Not Only a Silent Repressor but Also an Active Protector.Mol Neurobiol. 2017 Jan;54(1):541-550. doi: 10.1007/s12035-015-9658-4. Epub 2016 Jan 7. Mol Neurobiol. 2017. PMID: 26742529 Review.
-
RE-1 silencer of transcription/neural restrictive silencer factor modulates ectodermal patterning during Xenopus development.J Neurosci. 2006 Mar 8;26(10):2820-9. doi: 10.1523/JNEUROSCI.5037-05.2006. J Neurosci. 2006. PMID: 16525062 Free PMC article.
References
-
- Ballas N, Battaglioli E, Atouf F, Andrés ME, Chenoweth J, Anderson ME, Burger C, Moniwa M, Davie JR, Bowers WJ, Federoff HJ, Rose DW, Rosenfeld MG, Brehm P, Mandel G. Regulation of neuronal traits by a novel transcriptional complex. Neuron. 2001;31:353–365. - PubMed
-
- Chen ZF, Paquette AJ, Anderson DJ. NRSF/REST is required in vivo for repression of multiple neuronal target genes during embryogenesis. Nat Genet. 1998;20:136–142. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
