Caspase activation in hair cells of the mouse utricle exposed to neomycin
- PMID: 12351727
- PMCID: PMC6757801
- DOI: 10.1523/JNEUROSCI.22-19-08532.2002
Caspase activation in hair cells of the mouse utricle exposed to neomycin
Abstract
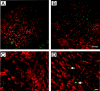
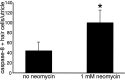
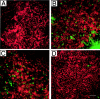
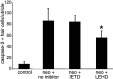
Aminoglycoside exposure results in the apoptotic destruction of auditory and vestibular hair cells. This ototoxic hair cell death is prevented by broad-spectrum caspase inhibition. We have used in situ substrate detection, immunohistochemistry, and specific caspase inhibitors to determine which caspases are activated in the hair cells of the adult mouse utricle in response to neomycin exposure in vitro. In addition, we have examined the hierarchy of caspase activation. Our data indicate that both upstream caspase-8 and upstream caspase-9, as well as downstream caspase-3 are activated in hair cells exposed to neomycin. The inhibition of caspase-9-like activity provided significant protection of hair cells exposed to neomycin, whereas the inhibition of caspase-8-like activity was not effective in preventing neomycin-induced hair cell death. In addition, caspase-9 inhibition prevented the activation of downstream caspase-3, whereas the inhibition of caspase-8 did not. These data indicate that caspase-9 is the primary upstream caspase mediating neomycin-induced hair cell death in this preparation.
Figures
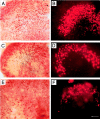
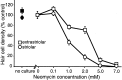
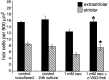
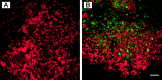


References
-
- Atlante A, Calissano P, Bobba A, Azzariti A, Marra E, Passarella S. Cytochrome c is released from mitochondria in a reactive oxygen species (ROS)-dependent fashion and can operate as a ROS scavenger and as a respiratory substrate in cerebellar neurons undergoing excitotoxic death. J Biol Chem. 2000;275:37159–37166. - PubMed
-
- Bagger-Sjoback D, Wersall J. Gentamicin-induced mitochondrial damage in inner ear sensory cells of the lizard Calotes versicolor. Acta Otolaryngol. 1978;86:35–51. - PubMed
-
- Bedner E, Smolewski P, Amstad P, Darzynkiewicz Z. Activation of caspases measured in situ by binding of fluorochrome-labeled inhibitors of caspases (FLICA): correlation with DNA fragmentation. Exp Cell Res. 2000;259:308–313. - PubMed
-
- Bhave SA, Oesterle EC, Coltrera MD. Macrophage and microglia-like cells in the avian inner ear. J Comp Neurol. 1998;398:241–256. - PubMed
-
- Bossy-Wetzel E, Green DR. Apoptosis: checkpoint at the mitochondrial frontier. Mutat Res. 1999;434:243–251. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
