Refinement of thalamocortical arbors and emergence of barrel domains in the primary somatosensory cortex: a study of normal and monoamine oxidase a knock-out mice
- PMID: 12351728
- PMCID: PMC6757778
- DOI: 10.1523/JNEUROSCI.22-19-08541.2002
Refinement of thalamocortical arbors and emergence of barrel domains in the primary somatosensory cortex: a study of normal and monoamine oxidase a knock-out mice
Abstract
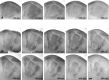
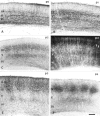
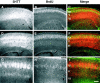
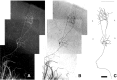
In the rodent primary somatosensory cortex, the thalamocortical axons (TCAs) are organized into clusters that correspond to functional units in the periphery. Around these axons, neurons in layer IV aggregate as barrels. To understand how this organization emerges, we analyzed TCA development in mice that do not form barrels, the monoamine oxidase A knock-out (MAOA-KO), and in MAOA/5-HT(1B) receptor double-KO mice, which have a restored barrel field. We show that TCAs already attain cortical layer IV on the day of birth. They are uniformly distributed in this layer from postnatal day 0 (P0) to P2 and secondarily coalesce into barrel domains in layer IV, over a 3 d period (P3-P5), with no prepatterning in the deeper layers. In MAOA-KO mice, the uniform distribution of the TC projection is maintained, and no axon clusters emerge. Individual TCA arbors were traced after carbocyanine injections. At P1, TCAs were poorly branched and covered variable tangential widths, encompassing one to two prospective barrels. At P7 the number of TCA branches increased 10-fold in layer IV and became restricted to one barrel. In MAOA-KO mice, there was a 50% reduction of the TCA terminal branches in layer IV, with a 40% increase in their tangential extent. These defects were corrected in the MAOA/5-HT(1B) double knock-out mice, indicating an effect of the presynaptic 5-HT(1B) receptor on axon branching. Our results indicate that the barrel-deficient phenotype of MAOA-KO mice results from an altered refinement of the TCA arbors in their target layer IV, involving branch elaboration and collateral retraction during early postnatal life.
Figures






References
-
- Agmon A, Connors BW. Thalamocortical responses of mouse somatosensory (barrel) cortex in vitro. Neuroscience. 1991;41:365–379. - PubMed
-
- Arnold PB, Li CX, Waters RS. Thalamocortical arbors extend beyond single cortical barrels: an in vivo intracellular tracing study in rat. Exp Brain Res. 2001;136:152–168. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
