Frizzled-3 is required for the development of major fiber tracts in the rostral CNS
- PMID: 12351730
- PMCID: PMC6757770
- DOI: 10.1523/JNEUROSCI.22-19-08563.2002
Frizzled-3 is required for the development of major fiber tracts in the rostral CNS
Abstract
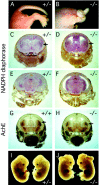
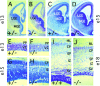
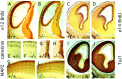
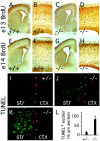
Many ligand/receptor families are known to contribute to axonal growth and targeting. Thus far, there have been no reports implicating Wnts and Frizzleds in this process, despite their large numbers and widespread expression within the CNS. In this study, we show that targeted deletion of the mouse fz3 gene leads to severe defects in several major axon tracts within the forebrain. In particular, fz3(-/-) mice show a complete loss of the thalamocortical, corticothalamic, and nigrostriatal tracts and of the anterior commissure, and they show a variable loss of the corpus callosum. Peripheral nerve fibers and major axon tracts in the more caudal regions of the CNS are mostly or completely unaffected. Cell proliferation in the ventricular zone and cell migration to the developing cortex proceed normally until at least embryonic day 14. Extensive cell death in the fz3(-/-) striatum occurs late in gestation, perhaps secondary to the nearly complete absence of long-range connections. In contrast, there is little cell death, as assayed by terminal deoxynucleotidyl transferase-mediated biotinylated UTP nick end labeling, in the cortex. These data provide the first link between Frizzled signaling and axonal development.
Figures








References
-
- Adler PN, Lee H. Frizzled signaling and cell-cell interactions in planar polarity. Curr Opin Cell Biol. 2001;13:635–640. - PubMed
-
- Auladell C, Perez-Sust P, Super H, Soriano E. The development of thalamocortical and corticothalamic projections in the mouse. Anat Embryol. 2000;201:169–179. - PubMed
-
- Bagri A, Marin O, Plump AS, Mak J, Pleasure SJ, Rubenstein JL, Tessier-Lavigne M. Slit proteins prevent midline crossing and determine the dorsoventral position of major axonal pathways in the mammalian forebrain. Neuron. 2002;33:233–248. - PubMed
-
- Bancroft JD, Stevens A. Theory and practice of histological techniques, pp 396–399. Churchill Livingstone; New York: 1982.
-
- Bayer SA, Altmann J. Neurogenesis and neuronal migration. In: Paxinos G, editor. The rat nervous system. Academic; New York: 1995. pp. 1041–1078.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
