Absence of fibroblast growth factor 2 promotes oligodendroglial repopulation of demyelinated white matter
- PMID: 12351731
- PMCID: PMC6757804
- DOI: 10.1523/JNEUROSCI.22-19-08574.2002
Absence of fibroblast growth factor 2 promotes oligodendroglial repopulation of demyelinated white matter
Abstract
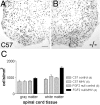
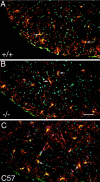
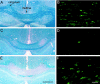
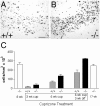
This study takes advantage of fibroblast growth factor 2 (FGF2) knock-out mice to determine the contribution of FGF2 to the regeneration of oligodendrocytes in the adult CNS. The role of FGF2 during spontaneous remyelination was examined using two complementary mouse models of experimental demyelination. The murine hepatitis virus strain A59 (MHV-A59) model produces focal areas of spinal cord demyelination with inflammation. The cuprizone neurotoxicant model causes extensive corpus callosum demyelination without a lymphocytic cell response. In both models, FGF2 expression is upregulated in areas of demyelination in wild-type mice. Surprisingly, in both models, oligodendrocyte repopulation of demyelinated white matter was significantly increased in FGF2 -/- mice compared with wild-type mice and even surpassed the oligodendrocyte density of nonlesioned mice. This dramatic result indicated that the absence of FGF2 promoted oligodendrocyte regeneration, possibly by enhancing oligodendrocyte progenitor proliferation and/or differentiation. FGF2 -/- and +/+ mice had similar oligodendrocyte progenitor densities in normal adult CNS, as well as similar progenitor proliferation and accumulation during demyelination. To directly analyze progenitor differentiation, glial cultures from spinal cords of wild-type mice undergoing remyelination after MHV-A59 demyelination were treated for 3 d with either exogenous FGF2 or an FGF2 neutralizing antibody. Elevating FGF2 favored progenitor proliferation, whereas attenuating endogenous FGF2 activity promoted the differentiation of progenitors into oligodendrocytes. These in vitro results are consistent with enhanced progenitor differentiation in FGF2 -/- mice. These studies demonstrate that the FGF2 genotype regulates oligodendrocyte regeneration and that FGF2 appears to inhibit oligodendrocyte lineage differentiation during remyelination.
Figures




References
-
- Allamargot C, Pouplard-Barthelaix A, Fressinaud C. A single intracerebral microinjection of platelet-derived growth factor (PDGF) accelerates the rate of remyelination in vivo. Brain Res. 2001;918:28–39. - PubMed
-
- Arnett HA, Mason J, Marino M, Suzuki K, Matsushima GK, Ting JP. TNF alpha promotes proliferation of oligodendrocyte progenitors and remyelination. Nat Neurosci. 2001;4:1116–1122. - PubMed
-
- Bansal R, Pfeiffer SE. Novel stage in the oligodendrocyte lineage defined by reactivity of progenitors with R-mAb prior to O1 anti-galactocerebroside. J Neurosci Res. 1992;32:309–316. - PubMed
-
- Bansal R, Pfeiffer SE. Regulation of oligodendrocyte differentiation by fibroblast growth factors. Adv Exp Med Biol. 1997;429:69–77. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
