Model of thalamocortical slow-wave sleep oscillations and transitions to activated States
- PMID: 12351744
- PMCID: PMC6757797
- DOI: 10.1523/JNEUROSCI.22-19-08691.2002
Model of thalamocortical slow-wave sleep oscillations and transitions to activated States
Abstract
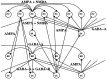
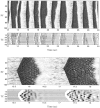
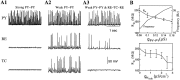
During natural slow-wave sleep (SWS) in nonanesthetized cats, silent (down) states alternate with active (up) states; the down states are absent during rapid-eye-movement sleep and waking. Oscillations (<1 Hz) in SWS and transformation to an activated awake state were investigated with intracellular recordings in vivo and with computational models of the corticothalamic system. Occasional summation of the miniature EPSPs during the hyperpolarized (silent) phase of SWS oscillation activated the persistent sodium current and depolarized the membrane of cortical pyramidal (PY) cells sufficiently for spike generation. In the model, this triggered the active phase, which was maintained by lateral PY-PY excitation and persistent sodium current. Progressive depression of the excitatory interconnections and activation of Ca2+-dependent K+ current led to termination of the 20-25 Hz activity after 500-1000 msec. Including thalamocortical (TC) and thalamic reticular neurons in the model increased the duration of the active epochs up to 1-1.5 sec and introduced waning spindle sequences. An increase in acetylcholine activity, which is associated with activated states, was modeled by the reduction in the K+ leak current in PY and TC cells and by a decrease in intracortical PY-PY synaptic conductances. These changes eliminated the hyperpolarizing phases of network activity and transformed cortical neurons to tonic firing at 15-20 Hz. During the transition from SWS to the activated state, the input resistance of cortical neurons gradually increased and, in a fully activated state, reached the same or even higher values as during silent phases of SWS oscillations. The model describes many essential features of SWS and activated states in the thalamocortical system as well as the transition between them.
Figures









References
-
- Abbott LF, Varela JA, Sen K, Nelson SB. Synaptic depression and cortical gain control. Science. 1997;275:220–224. - PubMed
-
- Achermann P, Borbely AA. Low-frequency (<1 Hz) oscillations in the human sleep electroencephalogram. Neuroscience. 1997;81:213–222. - PubMed
-
- Amzica F, Steriade M. Short- and long-range neuronal synchronization of the slow (<1 Hz) cortical oscillation. J Neurophysiol. 1995b;73:20–38. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
