Combined magnetic resonance imaging and spectroscopic imaging approach to molecular imaging of prostate cancer
- PMID: 12353259
- PMCID: PMC1978163
- DOI: 10.1002/jmri.10172
Combined magnetic resonance imaging and spectroscopic imaging approach to molecular imaging of prostate cancer
Abstract
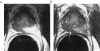
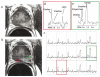
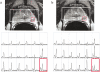
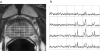
Magnetic resonance spectroscopic imaging (MRSI) provides a noninvasive method of detecting small molecular markers (historically the metabolites choline and citrate) within the cytosol and extracellular spaces of the prostate, and is performed in conjunction with high-resolution anatomic imaging. Recent studies in pre-prostatectomy patients have indicated that the metabolic information provided by MRSI combined with the anatomical information provided by MRI can significantly improve the assessment of cancer location and extent within the prostate, extracapsular spread, and cancer aggressiveness. Additionally, pre- and post-therapy studies have demonstrated the potential of MRI/MRSI to provide a direct measure of the presence and spatial extent of prostate cancer after therapy, a measure of the time course of response, and information concerning the mechanism of therapeutic response. In addition to detecting metabolic biomarkers of disease behavior and therapeutic response, MRI/MRSI guidance can improve tissue selection for ex vivo analysis. High-resolution magic angle spinning ((1)H HR-MAS) spectroscopy provides a full chemical analysis of MRI/MRSI-targeted tissues prior to pathologic and immunohistochemical analyses of the same tissue. Preliminary (1)H HR-MAS spectroscopy studies have already identified unique spectral patterns for healthy glandular and stromal tissues and prostate cancer, determined the composition of the composite in vivo choline peak, and identified the polyamine spermine as a new metabolic marker of prostate cancer. The addition of imaging sequences that provide other functional information within the same exam (dynamic contrast uptake imaging and diffusion-weighted imaging) have also demonstrated the potential to further increase the accuracy of prostate cancer detection and characterization.
Copyright 2002 Wiley-Liss, Inc.
Figures

References
-
- American Cancer Society . American Cancer Society Inc, Surveillance Research; 2002. Website: www.cancer.org.
-
- Han M, Partin AW, Piantadosi S, Epstein JI, Walsh PC. Era specific biochemical recurrence-free survival following radical prostatectomy for clinically localized prostate cancer. J Urol. 2001;166:416–419. - PubMed
-
- Stamey TA. Cancer of the prostate: an analysis of some important contributions and dilemmas. Mono Urol. 1982;3:67–94.
-
- McNeal JE, Kindrachuk RA, Freiha FS, Bostwick DG, Redwine EA, Stamey TA. Patterns of progression in prostate cancer. Lancet. 1986;1:60–63. - PubMed
-
- Quinlan DM, Partin AW, Walsh PC. Can aggressive prostatic carcinomas be identified and can their natural history be altered by treatment? Urology. 1995;46:77–82. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

