Genetic transformation of Vitis vinifera via organogenesis
- PMID: 12354328
- PMCID: PMC130035
- DOI: 10.1186/1472-6750-2-18
Genetic transformation of Vitis vinifera via organogenesis
Abstract
Background: Efficient transformation and regeneration methods are a priority for successful application of genetic engineering to vegetative propagated plants such as grape. The current methods for the production of transgenic grape plants are based on Agrobacterium-mediated transformation followed by regeneration from embryogenic callus. However, grape embryogenic calli are laborious to establish and the phenotype of the regenerated plants can be altered.
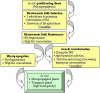
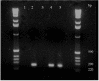
Results: Transgenic grape plants (V. vinifera, table-grape cultivars Silcora and Thompson Seedless) were produced using a method based on regeneration via organogenesis. In vitro proliferating shoots were cultured in the presence of increasing concentrations of N6-benzyl adenine. The apical dome of the shoot was removed at each transplantation which, after three months, produced meristematic bulk tissue characterized by a strong capacity to differentiate adventitious shoots. Slices prepared from the meristematic bulk were used for Agrobacterium-mediated transformation of grape plants with the gene DefH9-iaaM. After rooting on kanamycin containing media and greenhouse acclimatization, transgenic plants were transferred to the field. At the end of the first year of field cultivation, DefH9-iaaM grape plants were phenotypically homogeneous and did not show any morphological alterations in vegetative growth. The expression of DefH9-iaaM gene was detected in transgenic flower buds of both cultivars.
Conclusions: The phenotypic homogeneity of the regenerated plants highlights the validity of this method for both propagation and genetic transformation of table grape cultivars. Expression of the DefH9-iaaM gene takes place in young flower buds of transgenic plants from both grape cultivars.
Figures






References
-
- Barlass M, Skene KGM. In vitro propagation of grapevine (Vitis vinifera L.) from fragmented shoot apices. Vitis. 1978;17:335–340.
-
- Stamp JA, Meredith CP. Somatic embryogenesis from leaves and anthers of grapevine. Scientia Horticulturae. 1988;35:235–250. doi: 10.1016/0304-4238(88)90117-3. - DOI
-
- Mullins MG, Rajasekaran K. Plantlets from cultured anthers of Vitis species and hybrids. Proc Third Int Symp Grape Breeding, Davis. 1980. pp. 111–119.
-
- Stamp JA, Colby SM, Meredith CP. Direct shoot organogenesis and plant regeneration from leaves of grape (Vitis spp.). Plant Cell Tissue Organ Culture. 1990;22:127–133.
-
- Martinelli L, Bragagna P, Poletti V, Scienza A. Somatic embryogenesis from leaf- and petiole-derived callus of Vitis rupestris. Plant Cell Report. 1993;12:207–210. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

