Transmembrane collagen XVII, an epithelial adhesion protein, is shed from the cell surface by ADAMs
- PMID: 12356719
- PMCID: PMC129053
- DOI: 10.1093/emboj/cdf532
Transmembrane collagen XVII, an epithelial adhesion protein, is shed from the cell surface by ADAMs
Abstract
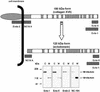
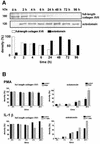
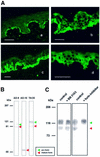
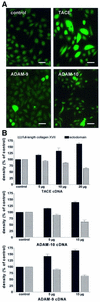
Collagen XVII, a type II transmembrane protein and epithelial adhesion molecule, can be proteolytically shed from the cell surface to generate a soluble collagen. Here we investigated the release of the ectodomain and identified the enzymes involved. After surface biotinylation of keratinocytes, the ectodomain was detectable in the medium within minutes and remained stable for >48 h. Shedding was enhanced by phorbol esters and inhibited by metalloprotease inhibitors, including hydroxamates and TIMP-3, but not by inhibitors of other protease classes or by TIMP-2. This profile implicated MMPs or ADAMs as candidate sheddases. MMP-2, MMP-9 and MT1-MMP were excluded, but TACE, ADAM-10 and ADAM-9 were shown to be expressed in keratinocytes and to be actively involved. Transfection with cDNAs for the three ADAMs resulted in increased shedding and, vice versa, in TACE-deficient cells shedding was significantly reduced, indicating that transmembrane collagen XVII represents a novel class of substrates for ADAMs. Functionally, release of the ectodomain of collagen XVII from the cell surface was associated with altered keratinocyte motility in vitro.
Figures



Similar articles
-
Shedding of collagen XVII ectodomain depends on plasma membrane microenvironment.J Biol Chem. 2005 Oct 7;280(40):34019-24. doi: 10.1074/jbc.M503751200. Epub 2005 Jul 14. J Biol Chem. 2005. PMID: 16020548
-
The metalloprotease-directed shedding of BP 180 (collagen XVII) from human keratinocytes in culture is unaffected by ceramide and cell-matrix interaction.Eur J Dermatol. 2002 May-Jun;12(3):240-6. Eur J Dermatol. 2002. PMID: 11978564
-
The shed ectodomain of collagen XVII/BP180 is targeted by autoantibodies in different blistering skin diseases.Am J Pathol. 2000 Feb;156(2):685-95. doi: 10.1016/S0002-9440(10)64772-4. Am J Pathol. 2000. PMID: 10666397 Free PMC article.
-
Collagenous transmembrane proteins: collagen XVII as a prototype.Matrix Biol. 2003 Jun;22(4):299-309. doi: 10.1016/s0945-053x(03)00051-9. Matrix Biol. 2003. PMID: 12935815 Review.
-
Gene therapy of epidermolysis bullosa.Expert Opin Biol Ther. 2004 Sep;4(9):1435-43. doi: 10.1517/14712598.4.9.1435. Expert Opin Biol Ther. 2004. PMID: 15335311 Review.
Cited by
-
The Neural Progenitor Cell-Associated Transcription Factor FoxG1 Regulates Cardiac Epicardial Cell Proliferation.Stem Cells Int. 2024 Jan 11;2024:8601360. doi: 10.1155/2024/8601360. eCollection 2024. Stem Cells Int. 2024. PMID: 38239823 Free PMC article.
-
Synergistic activation of p53 by actinomycin D and nutlin-3a is associated with the upregulation of crucial regulators and effectors of innate immunity.Cell Signal. 2020 May;69:109552. doi: 10.1016/j.cellsig.2020.109552. Epub 2020 Feb 4. Cell Signal. 2020. PMID: 32032660 Free PMC article.
-
ADAM function in embryogenesis.Semin Cell Dev Biol. 2009 Apr;20(2):153-63. doi: 10.1016/j.semcdb.2008.09.006. Epub 2008 Sep 30. Semin Cell Dev Biol. 2009. PMID: 18935966 Free PMC article. Review.
-
Bullous pemphigoid IgG induces BP180 internalization via a macropinocytic pathway.Am J Pathol. 2013 Mar;182(3):828-40. doi: 10.1016/j.ajpath.2012.11.029. Epub 2013 Jan 19. Am J Pathol. 2013. PMID: 23337823 Free PMC article.
-
Autocrine extracellular signal-regulated kinase (ERK) activation in normal human keratinocytes: metalloproteinase-mediated release of amphiregulin triggers signaling from ErbB1 to ERK.Mol Biol Cell. 2004 Sep;15(9):4299-309. doi: 10.1091/mbc.e04-03-0233. Epub 2004 Jul 14. Mol Biol Cell. 2004. PMID: 15254267 Free PMC article.
References
-
- Airola K., Ahonen,M., Johansson,N., Heikkila,P., Kere,J., Kahari,V.M. and Saarialho-Kere,U.K. (1998) Human TIMP-3 is expressed during fetal development, hair growth cycle, and cancer progression. J. Histochem. Cytochem., 46, 437–447. - PubMed
-
- Amour A. et al. (1998) TNF-α converting enzyme (TACE) is inhibited by TIMP-3. FEBS Lett., 435, 39–44. - PubMed
-
- Amour A., Knight,C.G., Webster,A., Slocombe,P.M., Stephens,P.E., Knauper,V., Docherty,A.J. and Murphy,G. (2000) The in vitro activity of ADAM-10 is inhibited by TIMP-1 and TIMP-3. FEBS Lett., 473, 275–279. - PubMed
-
- Angliker H., Neumann,U., Molloy,S.S. and Thomas,G. (1995) Internally quenched fluorogenic substrate for furin. Anal. Biochem., 224, 409–412. - PubMed
-
- Areida S.K., Reinhardt,D.P., Muller,P.K., Fietzek,P.P., Kowitz.J., Marinkovich,M.P. and Notbohm,H. (2001) Properties of the collagen type XVII ectodomain. Evidence for N- to C-terminal triple helix folding. J. Biol. Chem., 276, 1594–1601. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

