Two distinct phosphoinositide 3-kinases mediate polypeptide growth factor-stimulated PKB activation
- PMID: 12356726
- PMCID: PMC129034
- DOI: 10.1093/emboj/cdf512
Two distinct phosphoinositide 3-kinases mediate polypeptide growth factor-stimulated PKB activation
Abstract
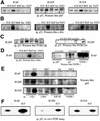
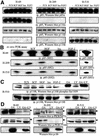
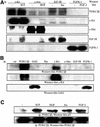
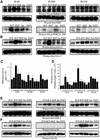
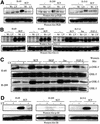
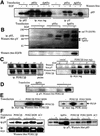
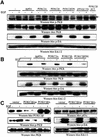
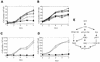
Eight human isoforms of phosphoinositide 3-kinases (PI3Ks) exist, but their individual functions remain poorly understood. Here, we show that different human small cell lung carcinoma (SCLC) cell lines overexpress distinct subsets of class I(A) and II PI3Ks, which results in striking differences in the signalling cascades activated by stem cell factor (SCF). Over expression of class I(A) p85/p110alpha in SCLC cells increased SCF-stimulated protein kinase B (PKB) activation and cell growth, but did not affect extracellular signal-regulated kinase (Erk) or glycogen synthase kinase-3 (GSK-3). This effect was selective, since it was not observed in SCLC cell lines overexpressing p85/p110beta or p85/p110delta. The SCF receptor associated with both class I(A) p85 and class II PI3KC2beta, and both enzymes contributed to SCF-stimulated PKB activity. A dominant-negative PI3KC2beta blocked both PKB activation and SCLC cell growth in response to SCF. Together our data provide novel insights into the specificity and functional significance of PI3K signalling in human cancer.
Figures



Similar articles
-
Critical role for lipid raft-associated Src kinases in activation of PI3K-Akt signalling.Cell Signal. 2007 May;19(5):1081-92. doi: 10.1016/j.cellsig.2006.12.003. Epub 2006 Dec 29. Cell Signal. 2007. PMID: 17275257
-
Evidence for functional redundancy of class IA PI3K isoforms in insulin signalling.Biochem J. 2007 Jun 15;404(3):449-58. doi: 10.1042/BJ20070003. Biochem J. 2007. PMID: 17362206 Free PMC article.
-
Cripto-1 induces phosphatidylinositol 3'-kinase-dependent phosphorylation of AKT and glycogen synthase kinase 3beta in human cervical carcinoma cells.Cancer Res. 1999 Sep 15;59(18):4502-5. Cancer Res. 1999. PMID: 10493495
-
The PI3K-PDK1 connection: more than just a road to PKB.Biochem J. 2000 Mar 15;346 Pt 3(Pt 3):561-76. Biochem J. 2000. PMID: 10698680 Free PMC article. Review.
-
A target for phosphoinositide 3-kinase: Akt/PKB.Trends Biochem Sci. 1995 Nov;20(11):441-2. doi: 10.1016/s0968-0004(00)89097-0. Trends Biochem Sci. 1995. PMID: 8578585 Review. No abstract available.
Cited by
-
Targeted expression of the class II phosphoinositide 3-kinase in Drosophila melanogaster reveals lipid kinase-dependent effects on patterning and interactions with receptor signaling pathways.Mol Cell Biol. 2004 Jan;24(2):796-808. doi: 10.1128/MCB.24.2.796-808.2004. Mol Cell Biol. 2004. PMID: 14701751 Free PMC article.
-
The Phosphatidylinositol 3-kinase/Akt Signaling Pathway in Neuroendocrine Tumors.Glob J Biochem. 2012;3:3. Epub 2011 Mar 8. Glob J Biochem. 2012. PMID: 27990410 Free PMC article. Review.
-
Receptor tyrosine kinase (c-Kit) inhibitors: a potential therapeutic target in cancer cells.Drug Des Devel Ther. 2016 Aug 1;10:2443-59. doi: 10.2147/DDDT.S89114. eCollection 2016. Drug Des Devel Ther. 2016. PMID: 27536065 Free PMC article. Review.
-
Phosphoinositide 3-kinase C2β regulates RhoA and the actin cytoskeleton through an interaction with Dbl.PLoS One. 2012;7(9):e44945. doi: 10.1371/journal.pone.0044945. Epub 2012 Sep 12. PLoS One. 2012. PMID: 22984590 Free PMC article.
-
Targeting the Mammalian Target of Rapamycin in Lung Cancer.Am J Med Sci. 2016 Nov;352(5):507-516. doi: 10.1016/j.amjms.2016.08.014. Epub 2016 Aug 21. Am J Med Sci. 2016. PMID: 27865299 Free PMC article. Review.
References
-
- Alessi D.R., James,S.R., Downes,C.P., Holmes,A.B., Gaffney,P., Reece,C. and Cohen,P. (1997) Purification and characterization of a phosphatidylinositol 3,4,5 trisphosphate-dependent protein kinase (PDK1) which phosphorylates and activates protein kinase Bα. Curr. Biol., 7, 261–269. - PubMed
-
- Alessi D.R., Kozlowski,M.T., Weng,Q.P., Morrice,N. and Avruch,J. (1998) 3-Phosphoinositide-dependent protein kinase 1 (PDK1) phosphorylates and activates the p70 S6 kinase in vivo and in vitro. Curr. Biol., 8, 69–81. - PubMed
-
- Arcaro A., Volinia,S., Zvelebil,M.J., Stein,R., Watton,S.J., Layton,M.J., Gout, I., Ahmadi, K., Downward,J. and Waterfield,M.D. (1998) Human phosphoinositide 3-kinase C2β, the role of calcium and the C2 domain in enzyme activity. J. Biol. Chem., 273, 33082–33090. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

