A novel vasopressin-induced transcript promotes MAP kinase activation and ENaC downregulation
- PMID: 12356727
- PMCID: PMC129031
- DOI: 10.1093/emboj/cdf509
A novel vasopressin-induced transcript promotes MAP kinase activation and ENaC downregulation
Abstract
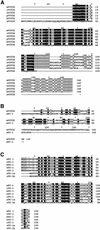
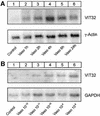
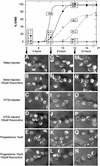
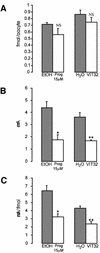
In the principal cell of the renal collecting duct, vasopressin regulates the expression of a gene network responsible for sodium and water reabsorption through the regulation of the water channel and the epithelial sodium channel (ENaC). We have recently identified a novel vasopressin-induced transcript (VIT32) that encodes for a 142 amino acid vasopressin-induced protein (VIP32), which has no homology with any protein of known function. The Xenopus oocyte expression system revealed two functions: (i) when injected alone, VIT32 cRNA rapidly induces oocyte meiotic maturation through the activation of the maturation promoting factor, the amphibian homolog of the universal M phase trigger Cdc2/cyclin; and (ii) when co-injected with the ENaC, VIT32 cRNA selectively downregulates channel activity, but not channel cell surface expression. In the kidney principal cell, VIP32 may be involved in the downregulation of transepithelial sodium transport observed within a few hours after vasopressin treatment. VIP32 belongs to a novel gene family ubiquitously expressed in oocyte and somatic cells that may be involved in G to M transition and cell cycling.
Figures




Similar articles
-
Long-term effects of vasopressin on the subcellular localization of ENaC in the renal collecting system.Kidney Int. 2006 Mar;69(6):1024-32. doi: 10.1038/sj.ki.5000211. Kidney Int. 2006. PMID: 16528252
-
Aldosterone induces rapid apical translocation of ENaC in early portion of renal collecting system: possible role of SGK.Am J Physiol Renal Physiol. 2001 Apr;280(4):F675-82. doi: 10.1152/ajprenal.2001.280.4.F675. Am J Physiol Renal Physiol. 2001. PMID: 11249859
-
Effects of the serine/threonine kinase SGK1 on the epithelial Na(+) channel (ENaC) and CFTR: implications for cystic fibrosis.Cell Physiol Biochem. 2001;11(4):209-18. doi: 10.1159/000051935. Cell Physiol Biochem. 2001. PMID: 11509829
-
Regulation of the epithelial sodium channel by accessory proteins.Biochem J. 2003 Apr 1;371(Pt 1):1-14. doi: 10.1042/BJ20021375. Biochem J. 2003. PMID: 12460120 Free PMC article. Review.
-
Molecular biology of Na+ absorption.Am J Physiol. 1997 Sep;273(3 Pt 1):G571-85. doi: 10.1152/ajpgi.1997.273.3.G571. Am J Physiol. 1997. PMID: 9316461 Review.
Cited by
-
Role of epithelial sodium channels and their regulators in hypertension.J Biol Chem. 2010 Oct 1;285(40):30363-9. doi: 10.1074/jbc.R110.155341. Epub 2010 Jul 12. J Biol Chem. 2010. PMID: 20624922 Free PMC article. Review.
-
Predicting patient outcomes with gene-expression biomarkers from colorectal cancer organoids and cell lines.Front Mol Biosci. 2025 Jan 15;12:1531175. doi: 10.3389/fmolb.2025.1531175. eCollection 2025. Front Mol Biosci. 2025. PMID: 39886381 Free PMC article.
-
Autophagy-prominent cell clusters among human lens epithelial cells: integrated single-cell RNA-sequencing analysis.BMC Ophthalmol. 2023 Apr 20;23(1):168. doi: 10.1186/s12886-023-02910-8. BMC Ophthalmol. 2023. PMID: 37081480 Free PMC article.
-
Acute downregulation of ENaC by EGF involves the PY motif and putative ERK phosphorylation site.J Gen Physiol. 2007 Sep;130(3):313-28. doi: 10.1085/jgp.200709775. J Gen Physiol. 2007. PMID: 17724164 Free PMC article.
-
Human Thyroid Cancer-1 (TC-1) is a vertebrate specific oncogenic protein that protects against copper and pro-apoptotic genes in yeast.Microb Cell. 2015 Jul 6;2(7):247-255. doi: 10.15698/mic2015.07.213. Microb Cell. 2015. PMID: 28357300 Free PMC article.
References
-
- Bankir L. (2001) Antidiuretic action of vasopressin: quantitative aspects and interaction between V1a and V2 receptor-mediated effects. Cardiovasc. Res., 51, 372–390. - PubMed
-
- Bens M., Vallet,V., Cluzeaud,F., Pascual-Letallec,L., Kahn,A., Rafestin-Oblin,M.E., Rossier,B.C. and Vandewalle,A. (1999) Corticosteroid-dependent sodium transport in a novel immortalized mouse collecting duct principal cell line. J. Am. Soc. Nephrol., 10, 923–934. - PubMed
-
- Canessa C.M., Schild,L., Buell,G., Thorens,B., Gautschi,I., Horisberger,J.D. and Rossier,B.C. (1994) Amiloride-sensitive epithelial Na+ channel is made of three homologous subunits. Nature, 367, 463–467. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

