The effects of HCl and CaCl(2) injections on intracellular calcium and pH in voltage-clamped snail (Helix aspersa) neurons
- PMID: 12356857
- PMCID: PMC2229535
- DOI: 10.1085/jgp.20028665
The effects of HCl and CaCl(2) injections on intracellular calcium and pH in voltage-clamped snail (Helix aspersa) neurons
Abstract
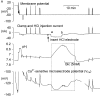
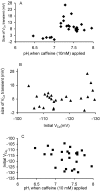
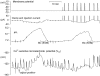
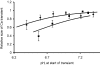
To investigate the mechanisms by which low intracellular pH influences calcium signaling, I have injected HCl, and in some experiments CaCl(2), into snail neurons while recording intracellular pH (pH(i)) and calcium concentration ([Ca(2+)](i)) with ion-sensitive microelectrodes. Unlike fluorescent indicators, these do not increase buffering. Slow injections of HCl (changing pH(i) by 0.1-0.2 pH units min(-1)) first decreased [Ca(2+)](i) while pH(i) was still close to normal, but then increased [Ca(2+)](i) when pH(i) fell below 6.8-7. As pH(i) recovered after such an injection, [Ca(2+)](i) started to fall but then increased transiently before returning to its preinjection level. Both the acid-induced decrease and the recovery-induced increase in [Ca(2+)](i) were abolished by cyclopiazonic acid, which empties calcium stores. Caffeine with or without ryanodine lowered [Ca(2+)](i) and converted the acid-induced fall in [Ca(2+)](i) to an increase. Injection of ortho-vanadate increased steady-state [Ca(2+)](i) and its response to acidification, which was again blocked by CPA. The normal initial response to 10 mM caffeine, a transient increase in [Ca(2+)](i), did not occur with pH(i) below 7.1. When HCl was injected during a series of short CaCl(2) injections, the [Ca(2+)](i) transients (recorded as changes in the potential (V(Ca)) of the Ca(2+)-sensitive microelectrode), were reduced by only 20% for a 1 pH unit acidification, as was the rate of recovery after each injection. Calcium transients induced by brief depolarizations, however, were reduced by 60% by a similar acidification. These results suggest that low pH(i) has little effect on the plasma membrane calcium pump (PMCA) but important effects on the calcium stores, including blocking their response to caffeine. Acidosis inhibits spontaneous calcium release via the RYR, and leads to increased store content which is unloaded when pH(i) returns to normal. Spontaneous release is enhanced by the rise in [Ca(2+)](i) caused by inhibiting the PMCA.
Figures








Similar articles
-
Effects of low doses of caffeine on [Ca2+]i in voltage-clamped snail (Helix aspersa) neurones.J Physiol. 1995 Nov 15;489 ( Pt 1)(Pt 1):19-28. doi: 10.1113/jphysiol.1995.sp021026. J Physiol. 1995. PMID: 8583402 Free PMC article.
-
The effect of calcium pump inhibitors on the response of intracellular calcium to caffeine in snail neurones.Cell Calcium. 2001 Jul;30(1):41-8. doi: 10.1054/ceca.2001.0209. Cell Calcium. 2001. PMID: 11396986
-
The effects of intracellular pH changes on resting cytosolic calcium in voltage-clamped snail neurones.J Physiol. 2001 Feb 1;530(Pt 3):405-16. doi: 10.1111/j.1469-7793.2001.0405k.x. J Physiol. 2001. PMID: 11158272 Free PMC article.
-
Calcium content of the endoplasmic reticulum of snail neurones releasable by caffeine.Cell Calcium. 2013 Feb;53(2):120-4. doi: 10.1016/j.ceca.2012.10.007. Epub 2012 Nov 29. Cell Calcium. 2013. PMID: 23199838
-
Calcium oscillations in neurons.Ciba Found Symp. 1995;188:210-23; discussion 223-34. doi: 10.1002/9780470514696.ch12. Ciba Found Symp. 1995. PMID: 7587619 Review.
Cited by
-
Acid-evoked Ca2+ signalling in rat sensory neurones: effects of anoxia and aglycaemia.Pflugers Arch. 2009 Nov;459(1):159-81. doi: 10.1007/s00424-009-0715-6. Pflugers Arch. 2009. PMID: 19806360 Free PMC article.
-
The plasma membrane calcium ATPase (PMCA) of neurones is electroneutral and exchanges 2 H+ for each Ca2+ or Ba2+ ion extruded.J Physiol. 2009 Jan 15;587(2):315-27. doi: 10.1113/jphysiol.2008.162453. Epub 2008 Dec 8. J Physiol. 2009. PMID: 19064619 Free PMC article.
-
Whole-cell recording of intracellular pH with silanized and oiled patch-type single or double-barreled microelectrodes.Pflugers Arch. 2003 Nov;447(2):259-65. doi: 10.1007/s00424-003-1162-4. Epub 2003 Aug 21. Pflugers Arch. 2003. PMID: 12937988
-
Dynamic and static calcium gradients inside large snail (Helix aspersa) neurones detected with calcium-sensitive microelectrodes.Cell Calcium. 2007 Apr;41(4):365-78. doi: 10.1016/j.ceca.2006.07.010. Epub 2006 Sep 8. Cell Calcium. 2007. PMID: 16962659 Free PMC article.
-
Intracellular calcium homeostasis changes induced in rat spinal cord neurons by extracellular acidification.Neurochem Res. 2003 Oct;28(10):1543-7. doi: 10.1023/a:1025626526410. Neurochem Res. 2003. PMID: 14570399
References
-
- Ammann, D., T. Bührer, U. Schefer, M. Müller, and W. Simon. 1987. Intracellular neutral carrier-based Ca2+ microelectrode with subnanomolar detection limit. Pflugers Arch. 409:223–228. - PubMed
-
- Ballanyi, K., and K. Kaila. 1998. Activity-evoked changes in intracellular pH. pH and Brain Function. K. Kaila and B.R. Ransom, editors. Wiley-Liss, New York. 291–308.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

