CD40, an extracellular receptor for binding and uptake of Hsp70-peptide complexes
- PMID: 12356871
- PMCID: PMC2173242
- DOI: 10.1083/jcb.200208083
CD40, an extracellular receptor for binding and uptake of Hsp70-peptide complexes
Abstract
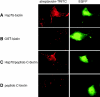
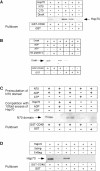
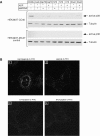
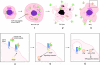
Tumor and viral antigens elicit a potent immune response by heat shock protein-dependent uptake of antigenic peptide with subsequent presentation by MHC I. Receptors on antigen-presenting cells that specifically bind and internalize a heat shock protein-peptide complex have not yet been identified. Here, we show that cells expressing CD40, a cell surface protein crucial for B cell function and autoimmunity, specifically bind and internalize human Hsp70 with bound peptide. Binding of Hsp70-peptide complex to the exoplasmic domain of CD40 is mediated by the NH(2)-terminal nucleotide-binding domain of Hsp70 in its ADP state. The Hsp70 cochaperone Hip, but not the bacterial Hsp70 homologue DnaK, competes formation of the Hsp70-CD40 complex. Binding of Hsp70-ADP to CD40 is strongly increased in the presence of Hsp70 peptide substrate, and induces signaling via p38. We suggest that CD40 is a cochaperone-like receptor mediating the uptake of exogenous Hsp70-peptide complexes by macrophages and dendritic cells.
Figures



References
-
- Arnold-Schild, D., D. Hanau, D. Spehner, C. Schmid, H.G. Rammensee, H. de la Salle, and H. Schild. 1999. Cutting edge: receptor-mediated endocytosis of heat shock proteins by professional antigen-presenting cells. J. Immunol. 162:3757–3760. - PubMed
-
- Asea, A., M. Rehli, E. Kabingu, J.A. Boch, O. Bare, P.E. Auron, M.A. Stevenson, and S.K. Calderwood. 2002. Novel signal transduction pathway utilized by extracellular HSP70: role of toll-like receptor (TLR) 2 and TLR4. J. Biol. Chem. 277:15028–15034. - PubMed
-
- Basu, S., R.J. Binder, R. Suto, K.M. Anderson, and P.K. Srivastava. 2000. Necrotic but not apoptotic cell death releases heat shock proteins, which deliver a partial maturation signal to dendritic cells and activate the NF-κB pathway. Int. Immunol. 12:1539–1546. - PubMed
-
- Berwin, B., and C.V. Nicchitta. 2001. To find the road traveled to tumor immunity: the trafficking itineraries of molecular chaperones in antigen-presenting cells. Traffic. 2:690–697. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

