Interactions of adenosine, prostaglandins and nitric oxide in hypoxia-induced vasodilatation: in vivo and in vitro studies
- PMID: 12356892
- PMCID: PMC2290577
- DOI: 10.1113/jphysiol.2002.023440
Interactions of adenosine, prostaglandins and nitric oxide in hypoxia-induced vasodilatation: in vivo and in vitro studies
Abstract
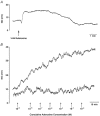
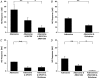
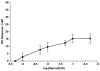
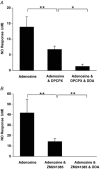
Adenosine, prostaglandins (PG) and nitric oxide (NO) have all been implicated in hypoxia-evoked vasodilatation. We investigated whether their actions are interdependent. In anaesthetised rats, the PG synthesis inhibitors diclofenac or indomethacin reduced muscle vasodilatation evoked by systemic hypoxia or adenosine, but not that evoked by iloprost, a stable analogue of prostacyclin (PGI(2)), or by an NO donor. After diclofenac, the A(1) receptor agonist CCPA evoked no vasodilatation: we previously showed that A(1), but not A(2A), receptors mediate the hypoxia-induced muscle vasodilatation. Further, in freshly excised rat aorta, adenosine evoked a release of NO, detected with an NO-sensitive electrode, that was abolished by NO synthesis inhibition, or endothelium removal, and reduced by ~50 % by the A(1) antagonist DPCPX, the remainder being attenuated by the A(2A) antagonist ZM241385. Diclofenac reduced adenosine-evoked NO release by ~50 % under control conditions, abolished that evoked in the presence of ZM241385, but did not affect that evoked in the presence of DPCPX. Adenosine-evoked NO release was also abolished by the adenyl cyclase inhibitor 2',5'-dideoxyadenosine, while dose-dependent NO release was evoked by iloprost. Finally, stimulation of A(1), but not A(2A), receptors caused a release of PGI(2) from rat aorta, assessed by radioimmunoassay of its stable metabolite, 6-keto PGF(1alpha), that was abolished by diclofenac. These results suggest that during systemic hypoxia, adenosine acts on endothelial A(1) receptors to increase PG synthesis, thereby generating cAMP, which increases the synthesis and release of NO and causes muscle vasodilatation. This pathway may be important in other situations involving these autocoids.
Figures
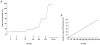



Comment in
-
Hypoxic vasodilatation: is an adenosine-prostaglandins- NO signalling cascade involved?J Physiol. 2002 Oct 1;544(Pt 1):2. doi: 10.1113/jphysiol.2002.028902. J Physiol. 2002. PMID: 12356875 Free PMC article.
Similar articles
-
Studies on the roles of ATP, adenosine and nitric oxide in mediating muscle vasodilatation induced in the rat by acute systemic hypoxia.J Physiol. 1996 Sep 1;495 ( Pt 2)(Pt 2):553-60. doi: 10.1113/jphysiol.1996.sp021615. J Physiol. 1996. PMID: 8887765 Free PMC article.
-
Cellular mechanisms by which adenosine induces vasodilatation in rat skeletal muscle: significance for systemic hypoxia.J Physiol. 1999 Jan 1;514 ( Pt 1)(Pt 1):163-75. doi: 10.1111/j.1469-7793.1999.163af.x. J Physiol. 1999. PMID: 9831724 Free PMC article.
-
ATP-induced vasodilation and purinergic receptors in the human leg: roles of nitric oxide, prostaglandins, and adenosine.Am J Physiol Regul Integr Comp Physiol. 2009 Apr;296(4):R1140-8. doi: 10.1152/ajpregu.90822.2008. Epub 2008 Dec 31. Am J Physiol Regul Integr Comp Physiol. 2009. PMID: 19118095
-
Adenosine and muscle vasodilatation in acute systemic hypoxia.Acta Physiol Scand. 2000 Apr;168(4):561-73. doi: 10.1046/j.1365-201x.2000.00709.x. Acta Physiol Scand. 2000. PMID: 10759593 Review.
-
Nitric oxide and prostaglandin systems in the stimulation of hypothalamic-pituitary-adrenal axis by neurotransmitters and neurohormones.J Physiol Pharmacol. 2004 Dec;55(4):679-703. J Physiol Pharmacol. 2004. PMID: 15613736 Review.
Cited by
-
Regulation of increased blood flow (hyperemia) to muscles during exercise: a hierarchy of competing physiological needs.Physiol Rev. 2015 Apr;95(2):549-601. doi: 10.1152/physrev.00035.2013. Physiol Rev. 2015. PMID: 25834232 Free PMC article. Review.
-
The Role of Hyperbaric Oxygen Therapy in Neuroregeneration and Neuroprotection: A Review.Cureus. 2024 Jun 10;16(6):e62067. doi: 10.7759/cureus.62067. eCollection 2024 Jun. Cureus. 2024. PMID: 38989389 Free PMC article. Review.
-
Of hemorrhagic shock, spherical cows and Aloe vera.Crit Care. 2004 Dec;8(6):406-7. doi: 10.1186/cc2975. Epub 2004 Oct 7. Crit Care. 2004. PMID: 15566601 Free PMC article.
-
Changes in muscle sympathetic nerve activity and vascular responses evoked in the spinotrapezius muscle of the rat by systemic hypoxia.J Physiol. 2011 May 1;589(Pt 9):2401-14. doi: 10.1113/jphysiol.2010.201814. Epub 2011 Feb 28. J Physiol. 2011. PMID: 21486771 Free PMC article.
-
Different Placebos, Different Mechanisms, Different Outcomes: Lessons for Clinical Trials.PLoS One. 2015 Nov 4;10(11):e0140967. doi: 10.1371/journal.pone.0140967. eCollection 2015. PLoS One. 2015. PMID: 26536471 Free PMC article. Clinical Trial.
References
-
- Akbar M, Okajima F, Tomura H, Shimegi S, Kondo Y. A single species of A1 adenosine receptor expressed in Chinese hamster ovary cells not only inhibits cAMP accumulation but also stimulates phospholipase-C and arachidonate release. Molecular Pharmacology. 1994;45:1036–1042. - PubMed
-
- Berne RM, Knabb RM, Ely SW, Rubio R. Adenosine in the local regulation of blood flow : a brief overview. Federation Proceedings. 1983;42:3136–3142. - PubMed
-
- Busse R, Forsterman U, Matsuda H, Pohl U. The role of prostaglandins in the endothelium-mediated vasodilatory response to hypoxia. Pflügers Archiv. 1984;401:77–83. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

