Length-dependent activation in three striated muscle types of the rat
- PMID: 12356894
- PMCID: PMC2290573
- DOI: 10.1113/jphysiol.2002.024505
Length-dependent activation in three striated muscle types of the rat
Abstract
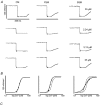
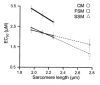
The process whereby sarcomere length modulates the sensitivity of the myofilaments to Ca(2+) is termed length-dependent activation. Length-dependent activation is a property of all striated muscles, yet the relative extent of length-dependent activation between skeletal muscle and cardiac muscle is unclear. Although length-dependent activation may be greater in fast skeletal muscle (FSM) than in slow skeletal muscle (SSM), there has not been a well controlled comparison of length-dependent activation between skeletal muscle and cardiac muscle (CM). Accordingly, we measured sarcomere length-dependent properties in skinned soleus (SSM), psoas (FSM) and ventricular trabeculae (CM) of the rat under carefully controlled conditions. The free Ca(2+)-force relationship was determined at sarcomere lengths (SL) of 1.95 microm, 2.10 microm and 2.25 microm and fitted to a modified Hill equation. FSM and SSM were more sensitive to Ca(2+) than CM. Length-dependent activation was ordered as CM > FSM > SSM. Cooperativity as measured by the Hill coefficient of the Ca(2+)-force relationship was not significantly different between CM and FSM, both of which exhibited greater cooperativity than SSM. SL did not significantly alter this parameter in each muscle type. To establish whether the observed differences can be explained by alterations in interfilament spacing, we measured myofilament lattice spacing (LS) by synchrotron X-ray diffraction in relaxed, skinned muscle preparations. LS was inversely proportional to SL for each muscle type. The slope of the SL-LS relationship, however, was not significantly different between striated muscle types. We conclude that (1) length-dependent activation differs among the three types of striated muscle and (2) these differences in the length-dependent properties among the striated muscle types may not solely be explained by the differences in the response of interfilament spacing to changes in muscle length in relaxed, skinned isolated muscle preparations.
Figures
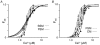

References
-
- Akella AB, Ding XL, Cheng R, Gulati J. Diminished Ca2+ sensitivity of skinned cardiac muscle contractility coincident with troponin T-band shifts in the diabetic rat. Circulation Research. 1995;76:600–606. - PubMed
-
- Allen DG, Kentish JC. The cellular basis of the length-tension relation in cardiac muscle. Journal of Molecular and Cellular Cardiology. 1985;17:821–840. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

