Pharmacological characterization of SB-710411 (Cpa-c[D-Cys-Pal-D-Trp-Lys-Val-Cys]-Cpa-amide), a novel peptidic urotensin-II receptor antagonist
- PMID: 12359626
- PMCID: PMC1573512
- DOI: 10.1038/sj.bjp.0704887
Pharmacological characterization of SB-710411 (Cpa-c[D-Cys-Pal-D-Trp-Lys-Val-Cys]-Cpa-amide), a novel peptidic urotensin-II receptor antagonist
Abstract
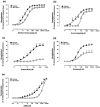
1. Human urotensin-II (hU-II), a cyclic undecapeptide, is amongst the most potent mammalian vasoconstrictors identified, suggesting that hU-II and its G-protein-coupled receptor (UT) may regulate cardiovascular homeostasis. Such a hypothesis would benefit greatly from the development of selective UT antagonists. 2. Although the somatostatin (SST) antagonist SB-710411 (Cpa-c[D-Cys-Pal-D-Trp-Lys-Val-Cys]-Cpa-amide) is purported to block U-II-induced contractions in rat isolated aorta, little is known about its specific pharmacological properties. 3. SB-710411 (10 micro M) inhibited hU-II-induced contraction in rat isolated aorta causing a significant, parallel shift in the agonist concentration-response curve (pK(b) 6.28+/-0.11; n=8) with no suppression of the E(max). In contrast, SB-710411 did not alter the contractile actions of angiotensin-II, phenylephrine, or KCl. Paradoxically, however, SB-710411 potentiated the contractile response to endothelin-1 (pEC(50) 8.02+/-0.16 and 8.54+/-0.11, P<0.01; n=8). Rather than being specific toSB-710411, this phenomenon appears to be related to somatostatin receptor affinity and not intrinsic activity since the SST agonist somatostatin-14 and antagonist cyclo-somatostatin also potentiated endothelin-1-induced contraction. 4. SB-710411 (10 micro M) did not inhibit carbachol, sodium nitroprusside, IBMX, isoprenaline, and levcromakalim-induced reversal of tone established with noradrenaline. In contrast, however, SB-710411 significantly inhibited the reversal of tone established with endothelin-1 using the same vasorelaxants. 5. In summary, although SB-710411 inhibits the vasoconstrictor actions of hU-II in a competitive, surmountable manner, it also possesses additional pharmacological actions. Thus, whilst the present study is amongst the first to detail the properties of a functional U-II receptor antagonist, the data suggest caution be used when assessing data generated utilizing this moiety and other SST analogues.
Figures



Similar articles
-
The peptidic urotensin-II receptor ligand GSK248451 possesses less intrinsic activity than the low-efficacy partial agonists SB-710411 and urantide in native mammalian tissues and recombinant cell systems.Br J Pharmacol. 2006 May;148(2):173-90. doi: 10.1038/sj.bjp.0706716. Br J Pharmacol. 2006. PMID: 16547525 Free PMC article.
-
Differential agonistic and antagonistic effects of the urotensin-II ligand SB-710411 at rodent and primate UT receptors.Eur J Pharmacol. 2004 May 25;492(2-3):113-6. doi: 10.1016/j.ejphar.2004.03.059. Eur J Pharmacol. 2004. PMID: 15178353
-
Nonpeptidic urotensin-II receptor antagonists I: in vitro pharmacological characterization of SB-706375.Br J Pharmacol. 2005 Jul;145(5):620-35. doi: 10.1038/sj.bjp.0706229. Br J Pharmacol. 2005. PMID: 15852036 Free PMC article.
-
Urotensin-II receptor peptide agonists.Med Res Rev. 2004 Sep;24(5):577-88. doi: 10.1002/med.20001. Med Res Rev. 2004. PMID: 15224381 Review.
-
Human urotensin-II, the most potent mammalian vasoconstrictor identified to date, as a therapeutic target for the management of cardiovascular disease.Trends Cardiovasc Med. 2000 Aug;10(6):229-37. doi: 10.1016/s1050-1738(00)00069-4. Trends Cardiovasc Med. 2000. PMID: 11282300 Review.
Cited by
-
Urotensin II modulates rapid eye movement sleep through activation of brainstem cholinergic neurons.J Neurosci. 2005 Jun 8;25(23):5465-74. doi: 10.1523/JNEUROSCI.4501-04.2005. J Neurosci. 2005. PMID: 15944374 Free PMC article.
-
Potential Clinical Implications of the Urotensin II Receptor Antagonists.Front Pharmacol. 2011 Jul 22;2:38. doi: 10.3389/fphar.2011.00038. eCollection 2011. Front Pharmacol. 2011. PMID: 21811463 Free PMC article.
-
Urotensin-ⅡReceptor Antagonist SB-710411 Protects Rat Heart against Ischemia-Reperfusion Injury via RhoA/ROCK Pathway.PLoS One. 2016 Jan 15;11(1):e0146094. doi: 10.1371/journal.pone.0146094. eCollection 2016. PLoS One. 2016. PMID: 26771557 Free PMC article.
-
Urantide: an ultrapotent urotensin II antagonist peptide in the rat aorta.Br J Pharmacol. 2003 Dec;140(7):1155-8. doi: 10.1038/sj.bjp.0705555. Br J Pharmacol. 2003. PMID: 14645137 Free PMC article.
-
Urotensin-II Ligands: An Overview from Peptide to Nonpeptide Structures.J Amino Acids. 2013;2013:979016. doi: 10.1155/2013/979016. Epub 2013 Feb 25. J Amino Acids. 2013. PMID: 23533711 Free PMC article.
References
-
- AMES R.S., SARAU H.M., CHAMBERS J.K., WILLETTE R.N., AIYAR N.V., ROMANIC A.M., LOUDEN C.S., FOLEY J.J., SAUERMELCH C.F., COATNEY R.W., AO Z., DISA J., HOLMES S.D., STADEL J.M., MARTIN J.D., LIU W.S., GLOVER G.I., WILSON S., MCNULTY D.E., ELLIS C.E., ELSHOURBAGY N.A., SHABON U., TRILL J.J., HAY D.W.P., OHLSTEIN E.H., BERGSMA D.J., DOUGLAS S.A. Human urotensin-II is a potent vasoconstrictor and agonist for the orphan receptor GPR14. Nature. 1999;401:282–286. - PubMed
-
- BEHM D.J., NASELSKY D.P., HEROLD C.L., AIYAR N.V., KNIGHT S.D., DHANAK D., DOUGLAS S.A. Pharmacological characterization of a novel peptidic urotensin-II receptor antagonist. Pharmacologist. 2001;43:190.
-
- CAMARDA V., RIZZI A., CALO G., GENDRON G., PERRON S.I., KOSTENIS E., ZAMBONI P., MASCOLI F., REGOLI D. Effects of human urotensin II in isolated vessels of various species; comparison with other vasoactive agents. Naunyn-Schmiedeberg's Arch. Pharmacol. 2002;365:141–149. - PubMed
-
- COY D.H., ROSSOWSKI W.J., CHENG B.L., HOCART S.J., TAYLOR J.E. Novel urotensin-II (UII) antagonists point to multiple receptor involvement in UII bioactivity. Reg. Pep. 2000;94:48.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

