Phenotypic reversion or death of cancer cells by altering signaling pathways in three-dimensional contexts
- PMID: 12359858
- PMCID: PMC2975573
- DOI: 10.1093/jnci/94.19.1494
Phenotypic reversion or death of cancer cells by altering signaling pathways in three-dimensional contexts
Abstract
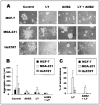
Background: We previously used a three-dimensional (3D) reconstituted basement membrane (rBM) assay to demonstrate that tumorigenic HMT-3522 T4-2 human breast cells can be induced to form morphologically normal structures ("reversion") by treatment with inhibitors of beta1 integrin, the epidermal growth factor receptor (EGFR), or mitogen-activated protein kinase (MAPK). We have now used this assay to identify reversion and/or death requirements of several more aggressive human breast cancer cell lines.
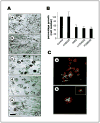
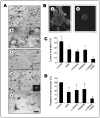
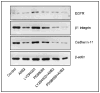
Methods: Breast tumor cell lines MCF7, Hs578T, and MDA-MB-231 were cultured in 3D rBM and treated with inhibitors of beta1 integrin, MAPK, or phosphatidylinositol 3-kinase (PI3K). MDA-MB-231 cells, which lack E-cadherin, were transfected with an E-cadherin cDNA. The extent of reversion was assessed by changes in morphology and polarity, growth in 3D rBM or soft agar, level of invasiveness, and tumor formation in nude mice.
Results: All three cell lines showed partial reversion (MCF7 the greatest and Hs578T the least) of tumorigenic properties treated with a single beta1 integrin, MAPK, or PI3K inhibitor. Combined inhibition of beta1 integrin and either PI3K or MAPK resulted in nearly complete phenotypic reversion (MDA-MB-231, MCF7) or in cell death (Hs578T). E-cadherin-transfected MDA-MB-231 cells showed partial reversion, but exposure of the transfectants to an inhibitor of beta1 integrin, PI3K, or MAPK led to nearly complete reversion.
Conclusion: The 3D rBM assay can be used to identify signaling pathways that, when manipulated in concert, can lead to the restoration of morphologically normal breast structures or to death of the tumor cells, even highly metastatic cells. This approach may be useful to design therapeutic intervention strategies for aggressive breast cancers.
Figures
References
-
- Bissell MJ, Weaver VM, Lelievre SA, Wang F, Petersen OW, Schmeichel KL. Tissue structure, nuclear organization, and gene expression in normal and malignant breast. Cancer Res. 1999;59(7 Suppl):1757s–63s. discussion 1763s–4s. - PubMed
-
- Gumbiner BM. Cell adhesion: the molecular basis of tissue architecture and morphogenesis. Cell. 1996;84:345–57. - PubMed
-
- Werb Z, Vu TH, Rinkenberger JL, Coussens LM. Matrix-degrading proteases and angiogenesis during development and tumor formation. APMIS. 1999;107:11–8. - PubMed
-
- Petersen OW, Ronnov-Jessen L, Howlett AR, Bissell MJ. Interaction with basement membrane serves to rapidly distinguish growth and differentiation pattern of normal and malignant human breast epithelial cells [published erratum appears in Proc Natl Acad Sci U S A 1993;90:2556] Proc Natl Acad Sci U S A. 1992;89:9064–8. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

