X-ray structure of a bifunctional protein kinase in complex with its protein substrate HPr
- PMID: 12359875
- PMCID: PMC129691
- DOI: 10.1073/pnas.192368699
X-ray structure of a bifunctional protein kinase in complex with its protein substrate HPr
Abstract
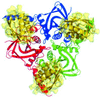
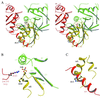
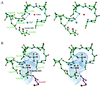
HPr kinase/phosphorylase (HprK/P) controls the phosphorylation state of the phosphocarrier protein HPr and regulates the utilization of carbon sources by Gram-positive bacteria. It catalyzes both the ATP-dependent phosphorylation of Ser-46 of HPr and its dephosphorylation by phosphorolysis. The latter reaction uses inorganic phosphate as substrate and produces pyrophosphate. We present here two crystal structures of a complex of the catalytic domain of Lactobacillus casei HprK/P with Bacillus subtilis HPr, both at 2.8-A resolution. One of the structures was obtained in the presence of excess pyrophosphate, reversing the phosphorolysis reaction and contains serine-phosphorylated HPr. The complex has six HPr molecules bound to the hexameric kinase. Two adjacent enzyme subunits are in contact with each HPr molecule, one through its active site and the other through its C-terminal helix. In the complex with serine-phosphorylated HPr, a phosphate ion is in a position to perform a nucleophilic attack on the phosphoserine. Although the mechanism of the phosphorylation reaction resembles that of eukaryotic protein kinases, the dephosphorylation by inorganic phosphate is unique to the HprK/P family of kinases. This study provides the structure of a protein kinase in complex with its protein substrate, giving insights into the chemistry of the phospho-transfer reactions in both directions.
Figures
Similar articles
-
Pyrophosphate-producing protein dephosphorylation by HPr kinase/phosphorylase: a relic of early life?Proc Natl Acad Sci U S A. 2002 Oct 15;99(21):13442-7. doi: 10.1073/pnas.212410399. Epub 2002 Oct 1. Proc Natl Acad Sci U S A. 2002. PMID: 12359880 Free PMC article.
-
Structural analysis of the bacterial HPr kinase/phosphorylase V267F mutant gives insights into the allosteric regulation mechanism of this bifunctional enzyme.J Biol Chem. 2007 Nov 30;282(48):34952-7. doi: 10.1074/jbc.M705979200. Epub 2007 Sep 18. J Biol Chem. 2007. PMID: 17878158
-
Phosphorylation of HPr by the bifunctional HPr Kinase/P-ser-HPr phosphatase from Lactobacillus casei controls catabolite repression and inducer exclusion but not inducer expulsion.J Bacteriol. 2000 May;182(9):2582-90. doi: 10.1128/JB.182.9.2582-2590.2000. J Bacteriol. 2000. PMID: 10762262 Free PMC article.
-
HPr kinase/phosphorylase, a Walker motif A-containing bifunctional sensor enzyme controlling catabolite repression in Gram-positive bacteria.Biochim Biophys Acta. 2004 Mar 11;1697(1-2):123-35. doi: 10.1016/j.bbapap.2003.11.018. Biochim Biophys Acta. 2004. PMID: 15023355 Review.
-
Transcription regulators potentially controlled by HPr kinase/phosphorylase in Gram-negative bacteria.J Mol Microbiol Biotechnol. 2003;5(4):206-15. doi: 10.1159/000071072. J Mol Microbiol Biotechnol. 2003. PMID: 12867744 Review.
Cited by
-
Effect of HPr phosphorylation on structure, dynamics, and interactions in the course of transcriptional control.J Mol Model. 2007 Mar;13(3):431-44. doi: 10.1007/s00894-006-0162-7. Epub 2006 Dec 1. J Mol Model. 2007. PMID: 17139481
-
Transporters of glucose and other carbohydrates in bacteria.Pflugers Arch. 2020 Sep;472(9):1129-1153. doi: 10.1007/s00424-020-02379-0. Epub 2020 May 6. Pflugers Arch. 2020. PMID: 32372286 Review.
-
Nutritional control of antibiotic resistance via an interface between the phosphotransferase system and a two-component signaling system.Antimicrob Agents Chemother. 2014;58(2):957-65. doi: 10.1128/AAC.01919-13. Epub 2013 Nov 25. Antimicrob Agents Chemother. 2014. PMID: 24277024 Free PMC article.
-
Comparison of tertiary structures of proteins in protein-protein complexes with unbound forms suggests prevalence of allostery in signalling proteins.BMC Struct Biol. 2012 May 3;12:6. doi: 10.1186/1472-6807-12-6. BMC Struct Biol. 2012. PMID: 22554255 Free PMC article.
-
The Lactobacillus casei ptsHI47T mutation causes overexpression of a LevR-regulated but RpoN-independent operon encoding a mannose class phosphotransferase system.J Bacteriol. 2004 Jul;186(14):4543-55. doi: 10.1128/JB.186.14.4543-4555.2004. J Bacteriol. 2004. PMID: 15231787 Free PMC article.
References
-
- Hunter T. (2000) Cell 100, 113-127. - PubMed
-
- Saier M. H., Wu, L. F. & Reizer, J. (1990) Trends Biochem. Sci. 15, 391-395. - PubMed
-
- Stülke J. & Hillen, W. (2000) Annu. Rev. Microbiol. 54, 849-880. - PubMed
-
- Deutscher J., Galinier, A. & Martin-Verstraete, I. (2001) in Bacillus subtilis and Its Closest Relatives: From Genes to Cells, eds. Sonenschein, A. L., Hoch, J. A. & Losick, R. (Am. Soc. Microbiol., Washington, DC), pp. 129–150.
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

