Hygienic hand antiseptics: should they not have activity and label claims against viruses?
- PMID: 12360145
- PMCID: PMC7172183
- DOI: 10.1067/mic.2002.124532
Hygienic hand antiseptics: should they not have activity and label claims against viruses?
Abstract
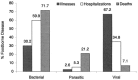
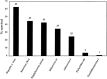
Enteric and respiratory viruses are among the most frequent causes of human infections, and hands play an important role in the spread of these and many other viral diseases. Regular and proper hand hygiene by caregivers and food handlers in particular is essential to decontaminate hands and potentially interrupt such spread. What would be considered a proper decontamination of hands? Handwashing with regular soap and water is often considered sufficient, but what of hygienic handwash and handrub antiseptic products? Are they more effective? The evidence suggests that some clearly are. Activity against bacteria may not reflect the ability of hygienic hand antiseptics to deal with viruses, especially those that are nonenveloped. In spite of the acknowledged importance of hands as vehicles for viruses, there is a lack of suitable regulatory mechanism for handwash or handrub products to make claims of efficacy against viruses. This is in contrast with the ability of general-purpose disinfectants to make antiviral claims, although transmission of viruses from surfaces other than those of reusable medical devices may play only a minor role in virus transmission. This review discusses the (1). recent information on the relative importance of viruses as human pathogens, particularly those causing enteric and respiratory infections; (2). the survival of relevant viruses on human hands in comparison with common gram-negative and gram-positive bacteria; (3). the potential of hands to transfer or receive such contamination on casual contact; (4). role of hands in the spread of viruses; (5). the potential of hygienic measures to eliminate viruses from contaminated hands; (6). relative merits of available protocols to assess the activity of hygienic hand antiseptics against viruses; and (7). factors considered crucial in any tests to assess the activity of hygienic hand antiseptics against viruses. In addition, this review proposes surrogate viruses in such testing and discusses issues for additional consideration by researchers, manufacturers, end-users, and regulators.
Figures
References
-
- Larson EL. APIC guideline for handwashing and hand antisepsis in health care settings. Am J Infect Control. 1995;23:251–269. - PubMed
-
- Health Canada Infection control guidelines: hand washing, cleaning, disinfection, and sterilization in health care. Can Commu Dis Rep. 1998;24(Suppl 8):1–55. - PubMed
-
- Garner J, Favero MS. : Centers for Disease Control and Prevention; Atlanta (GA): 1985. Guidelines for handwashing and hospital environmental control; pp. 1–20.
-
- US Food and Drug Administration . : US FDA; Washington: 1994. Topical antimicrobial drug products for over-the-counter human use. Tentative final monograph for health care antiseptic products. Federal register, code of federal regulations, parts 333 and 369, vol 59, no 116; pp. 31402–31452.
-
- Knipe DM, Howley PM, Griffin DE, Lamb RA, Martin MA, Roizman B. 4th ed. : Lippincott Williams & Wilkins; New York: 2001. Virology.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical