Protonation of non-Watson-Crick base pairs and encapsidation of turnip yellow mosaic virus RNA
- PMID: 12361978
- PMCID: PMC129696
- DOI: 10.1073/pnas.202287499
Protonation of non-Watson-Crick base pairs and encapsidation of turnip yellow mosaic virus RNA
Abstract
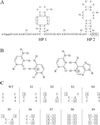
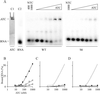
The 5' UTR of turnip yellow mosaic virus RNA contains two conserved hairpins with internal loops consisting of C.C and C.A mismatches. In this article, evidence is presented indicating that the 5' proximal hairpin functions as an encapsidation initiation signal. Extensive mutagenesis studies on this hairpin and sequencing of virus progeny showed a clear preference for C.C and C.A mismatches within the internal loop. The importance of these mismatches lies in their pH-dependent protonation and stable base pair formation. Encapsidation efficiency was found to be severely affected for several mutants lacking the protonatable mismatches in the internal loop of the 5' proximal hairpin. Furthermore, gel mobility-shift assays were performed with various RNA hairpins and empty capsids with a hole. Protonatable hairpins containing C.C and/or C.A pairs were found to bind specifically to the interior of the protein shell under acidic conditions (pH 4.5) in the presence of spermidine. Based on these results we propose that this binding of protonated cytosines to the coat protein of turnip yellow mosaic virus may represent a new motif in RNA-protein interactions.
Figures



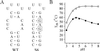
References
-
- Canady M A, Larson S B, Day J, McPherson A. Nat Struct Biol. 1996;3:771–781. - PubMed
-
- Hellendoorn K. Ph.D. thesis. Leiden, The Netherlands: Leiden University; 1997.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

