Diagnosis and management of human cytomegalovirus infection in the mother, fetus, and newborn infant
- PMID: 12364375
- PMCID: PMC126858
- DOI: 10.1128/CMR.15.4.680-715.2002
Diagnosis and management of human cytomegalovirus infection in the mother, fetus, and newborn infant
Abstract
Human cytomegalovirus (HCMV) is the leading cause of congenital viral infection and mental retardation. HCMV infection, while causing asymptomatic infections in most immunocompetent subjects, can be transmitted during pregnancy from the mother with primary (and also recurrent) infection to the fetus. Hence, careful diagnosis of primary infection is required in the pregnant woman based on the most sensitive serologic assays (immunoglobulin M [IgM] and IgG avidity assays) and conventional virologic and molecular procedures for virus detection in blood. Maternal prognostic markers of fetal infection are still under investigation. If primary infection is diagnosed in a timely manner, prenatal diagnosis can be offered, including the search for virus and virus components in fetal blood and amniotic fluid, with fetal prognostic markers of HCMV disease still to be defined. However, the final step for definite diagnosis of congenital HCMV infection is detection of virus in the blood or urine in the first 1 to 2 weeks of life. To date, treatment of congenital infection with antiviral drugs is only palliative both prior to and after birth, whereas the only efficacious preventive measure seems to be the development of a safe and immunogenic vaccine, including recombinant, subunit, DNA, and peptide-based vaccines now under investigation. The following controversial issues are discussed in the light of the most recent advances in the field: the actual perception of the problem; universal serologic screening before pregnancy; the impact of correct counseling on decision making by the couple involved; the role of prenatal diagnosis in ascertaining transmission of virus to the fetus; the impact of preconceptional and periconceptional infections on the prevalence of congenital infection; and the prevalence of congenitally infected babies born to mothers who were immune prior to pregnancy compared to the number born to mothers undergoing primary infection during pregnancy.
Figures



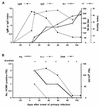





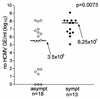
References
-
- Adler, S. P., S. A. Plotkin, E. Gonczol, M. Cadoz, C. Meric, J. B. Wang, P. Dellamonica, A. M. Best, J. Zahradnik, S. Pincus, K. Berencsi, W. I. Cox, and Z. Gyulai. 1999. A canarypox vector expressing cytomegalovirus (CMV) glycoprotein B primes for antibody responses to a live attenuated CMV vaccine (Towne). J. Infect. Dis. 180:843-846. - PubMed
-
- Ahlfors, K., M. Forsgren, S. A. Ivarsson, S. Harris, and L. Svanberg. 1983. Congenital cytomegalovirus infection: on the relation between type and time of maternal infection and infant's symptoms. Scand. J. Infect. Dis. 15:129-138. - PubMed
-
- Ahlfors, K., S. A. Ivarsson, and H. Nilsson. 1988. On the unpredictable development of congenital cytomegalovirus infection. A study in twins. Early Hum. Dev. 18:125-135. - PubMed
-
- Ahlfors, K., S. A. Ivarsson, S. Harris, L. Svanberg, R. Holmqvist, B. Lernmark, and G. Theander. 1984. Congenital cytomegalovirus infection and disease in Sweden and the relative importance of primary and secondary maternal infections. Preliminary findings from a prospective study. Scand. J. Infect. Dis. 16:129-137. - PubMed
-
- Ahlfors, K., S. A. Ivarsson, and S. Harris. 1999. Report on a long-term study of maternal and congenital cytomegalovirus infection in Sweden. Review of prospective studies available in the literature. Scand. J. Infect. Dis. 31:443-457. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

