Having it both ways: transcription factors that bind DNA and RNA
- PMID: 12364590
- PMCID: PMC140532
- DOI: 10.1093/nar/gkf512
Having it both ways: transcription factors that bind DNA and RNA
Abstract
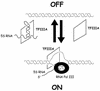
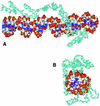
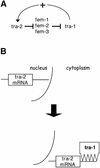
Multifunctional proteins challenge the conventional 'one protein-one function' paradigm. Here we note apparent multifunctional proteins with nucleic acid partners, tabulating eight examples. We then focus on eight additional cases of transcription factors that bind double-stranded DNA with sequence specificity, but that also appear to lead alternative lives as RNA-binding proteins. Exemplified by the prototypic Xenopus TFIIIA protein, and more recently by mammalian p53, this list of transcription factors includes WT-1, TRA-1, bicoid, the bacterial sigma(70) subunit, STAT1 and TLS/FUS. The existence of transcription factors that bind both DNA and RNA provides an interesting puzzle. Little is known concerning the biological roles of these alternative protein-nucleic acid interactions, and even less is known concerning the structural basis for dual nucleic acid specificity. We discuss how these natural examples have motivated us to identify artificial RNA sequences that competitively inhibit a DNA-binding transcription factor not known to have a natural RNA partner. The identification of such RNAs raises the possibility that RNA binding by DNA-binding proteins is more common than currently appreciated.
Figures


References
-
- Peyman J.A. (1999) Repression of major histocompatibility complex genes by a human trophoblast ribonucleic acid. Biol. Reprod., 60, 23–31. - PubMed
-
- Peyman J.A. (2001) Mammalian expression cloning of two human trophoblast suppressors of major histocompatibility complex genes. Am. J. Reprod. Immunol., 45, 382–392. - PubMed
-
- Perrotti D., Bonatti,S., Trotta,R., Martinez,R., Skorski,T., Salomoni,P., Grassilli,E., Iozzo,R.V., Cooper,D.R. and Calabretta,B. (1998) TLS/FUS, a pro-oncogene involved in multiple chromosomal translocations, is a novel regulator of BCR/ABL-mediated leukemogenesis. EMBO J., 17, 4442–4455. - PMC - PubMed
-
- Lerga A., Hallier,M., Delva,L., Orvain,C., Gallais,I., Marie,J. and Moreau-Gachelin,F. (2001) Identification of an RNA binding specificity for the potential splicing factor TLS. J. Biol. Chem., 276, 6807–6816. - PubMed
-
- Sakonju S., Bogenhagen,D.F. and Brown,D.D. (1980) A control region in the center of the 5S RNA gene directs specific initiation of transcription: 1. The 5′ border of the region. Cell, 19, 13–25. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

