Isomerization and oxidation of vitamin a in cone-dominant retinas: a novel pathway for visual-pigment regeneration in daylight
- PMID: 12367507
- PMCID: PMC2851622
- DOI: 10.1016/s0896-6273(02)00912-1
Isomerization and oxidation of vitamin a in cone-dominant retinas: a novel pathway for visual-pigment regeneration in daylight
Abstract
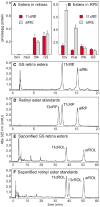
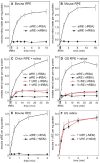
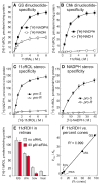
The first step toward light perception is 11-cis to all-trans photoisomerization of the retinaldehyde chromophore in a rod or cone opsin-pigment molecule. Light sensitivity of the opsin pigment is restored through a multistep pathway called the visual cycle, which effects all-trans to 11-cis re-isomerization of the retinoid chromophore. The maximum throughput of the known visual cycle, however, is too slow to explain sustained photosensitivity in bright light. Here, we demonstrate three novel enzymatic activities in cone-dominant ground-squirrel and chicken retinas: an all-trans-retinol isomerase, an 11-cis-retinyl-ester synthase, and an 11-cis-retinol dehydrogenase. Together these activities comprise a novel pathway that regenerates opsin photopigments at a rate 20-fold faster than the known visual cycle. We suggest that this pathway is responsible for sustained daylight vision in vertebrates.
Figures



Comment in
-
Like night and day: rods and cones have different pigment regeneration pathways.Neuron. 2002 Sep 26;36(1):1-3. doi: 10.1016/s0896-6273(02)00937-6. Neuron. 2002. PMID: 12367498 Review.
References
-
- Adler AJ, Spencer SA. Effect of light on endogenous ligands carried by interphotoreceptor retinoid-binding protein. Exp Eye Res. 1991;53:337–346. - PubMed
-
- Bok D, Ong DE, Chytil F. Immunocytochemical localization of cellular retinol binding protein in the rat retina. Invest Ophthalmol Vis Sci. 1984;25:877–883. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

