Different pattern of allelic loss in Epstein-Barr virus-positive gastric cancer with emphasis on the p53 tumor suppressor pathway
- PMID: 12368194
- PMCID: PMC1867286
- DOI: 10.1016/S0002-9440(10)64397-0
Different pattern of allelic loss in Epstein-Barr virus-positive gastric cancer with emphasis on the p53 tumor suppressor pathway
Abstract
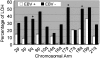
Both Helicobacter pylori (HP) and Epstein-Barr virus (EBV) have been implicated in carcinogenesis of the stomach. Fifty-seven gastric carcinomas were tested for microsatellite instability and allelic loss at several tumor suppressor loci using 21 polymorphic microsatellite markers. Furthermore, immunohistochemistry for p53 and DPC4/SMAD4 was performed. Results were analyzed according to HP and EBV status of the tumors, as assessed by immunohistochemistry and RNA in situ hybridization, respectively. Fractional allelic loss was lower in EBV-positive carcinomas (n = 15) when compared to EBV-negative carcinomas (P < 0.001). EBV positivity was inversely associated with allelic loss at specific markers on chromosomal arms 5q (APC), 17p (TP53), and 18q (DPC4/SMAD4). Allelic loss at the TP53 locus was not encountered in EBV-positive carcinomas, but occurred in 51% of EBV-negative carcinomas (P < 0.005). Moreover, none of the EBV-positive carcinomas showed unequivocal p53 immunopositivity in contrast to 39% of the EBV-negative carcinomas (P < 0.01). EBV-status was not related to microsatellite instability. There was no correlation between HP-status and any of the molecular alterations tested. In conclusion, EBV-positive gastric carcinomas follow a distinct pathogenesis at the molecular level, in which p53 is not, or differently inactivated.
Figures


Similar articles
-
Epstein-Barr virus-associated gastric carcinomas: relation to H. pylori infection and genetic alterations.Gastroenterology. 2000 Jun;118(6):1031-8. doi: 10.1016/s0016-5085(00)70355-6. Gastroenterology. 2000. PMID: 10833477
-
Epstein-Barr virus-associated gastric lymphomas are distinct from mucosa-associated lymphoid tissue-type lymphomas: genetic abnormalities of p53 gene.Diagn Mol Pathol. 2001 Sep;10(3):153-60. doi: 10.1097/00019606-200109000-00002. Diagn Mol Pathol. 2001. PMID: 11552717
-
Infrequent overexpression of p53 protein in Epstein-Barr virus-associated gastric carcinomas.Jpn J Cancer Res. 1997 Mar;88(3):262-6. doi: 10.1111/j.1349-7006.1997.tb00376.x. Jpn J Cancer Res. 1997. PMID: 9140110 Free PMC article.
-
[Epstein-Barr virus associated gastric carcinoma: the genetic alteration and the expression of CD44 variant].Nihon Rinsho. 1997 Feb;55(2):381-5. Nihon Rinsho. 1997. PMID: 9046827 Review. Japanese.
-
Epstein-Barr virus-associated lymphoepithelioma-like gastric carcinoma.Arch Pathol Lab Med. 2008 Apr;132(4):706-9. doi: 10.5858/2008-132-706-EVLGC. Arch Pathol Lab Med. 2008. PMID: 18384225 Review.
Cited by
-
EBV-infection in cardiac and non-cardiac gastric adenocarcinomas is associated with promoter methylation of p16, p14 and APC, but not hMLH1.Cell Oncol (Dordr). 2011 Jun;34(3):209-14. doi: 10.1007/s13402-011-0028-6. Epub 2011 May 3. Cell Oncol (Dordr). 2011. PMID: 21538028
-
Molecule action mechanisms of NM-3 on human gastric cancer SGC-7901 cells in vivo or in vitro.World J Gastroenterol. 2003 Oct;9(10):2366-9. doi: 10.3748/wjg.v9.i10.2366. World J Gastroenterol. 2003. PMID: 14562415 Free PMC article.
-
Epstein-Barr virus and gastric carcinoma--viral carcinogenesis through epigenetic mechanisms.Int J Clin Exp Pathol. 2008 Jan 1;1(3):198-216. Int J Clin Exp Pathol. 2008. PMID: 18784828 Free PMC article.
-
Epstein-Barr virus and nasopharyngeal carcinoma.Chin J Cancer. 2014 Dec;33(12):581-90. doi: 10.5732/cjc.014.10197. Epub 2014 Nov 21. Chin J Cancer. 2014. PMID: 25418193 Free PMC article.
-
Relationship between Epstein-Barr virus-encoded proteins with cell proliferation, apoptosis, and apoptosis-related proteins in gastric carcinoma.World J Gastroenterol. 2005 Jun 7;11(21):3234-9. doi: 10.3748/wjg.v11.i21.3234. World J Gastroenterol. 2005. PMID: 15929173 Free PMC article.
References
-
- Stadtländer CT, Waterbor JW: Molecular epidemiology, pathogenesis and prevention of gastric cancer. Carcinogenesis 1999, 20:2195-2208 - PubMed
-
- Laurén P: The two main types of gastric carcinoma: diffuse and so-called intestinal-type carcinomas: an attempt at a histo-clinical classification. Acta Pathol Microbiol Scand 1965, 64:31-49 - PubMed
-
- Schlemper RJ, Riddell RH, Kato Y, Borchard F, Cooper HS, Dawsey SM, Dixon MF, Fenoglio-Preiser CM, Flejou JF, Geboes K, Hattori T, Hirota T, Itabashi M, Iwafuchi M, Iwashita A, Kim YI, Kirchner T, Klimpfinger M, Koike M, Lauwers GY, Lewin KJ, Oberhuber G, Offner F, Price AB, Rubio CA, Shimizu M, Shimoda T, Sipponen P, Solcia E, Stolte M, Watanabe H, Yamabe H: The Vienna classification of gastrointestinal epithelial neoplasia. Gut 2000, 47:251-255 - PMC - PubMed
-
- Solcia E, Fiocca R, Luinetti O, Villani L, Padovan L, Calistri D, Ranzani GN, Chiaravalli A, Capella C: Intestinal and diffuse gastric cancers arise in a different background of Helicobacter pylori gastritis through different gene involvement. Am J Surg Pathol 1996, 20:S8-S22 - PubMed
-
- Fearon ER, Vogelstein B: A genetic model for colorectal tumorigenesis. Cell 1990, 61:759-767 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

