Virus-induced demyelination in nude mice is mediated by gamma delta T cells
- PMID: 12368199
- PMCID: PMC1867296
- DOI: 10.1016/s0002-9440(10)64402-1
Virus-induced demyelination in nude mice is mediated by gamma delta T cells
Abstract
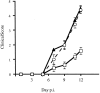
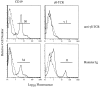
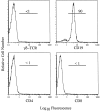
Infection of mice with mouse hepatitis virus (MHV), strain JHM, results in acute and chronic demyelination with many similarities to the human disease multiple sclerosis. This pathological process is primarily T cell-mediated and MHV infection of mice lacking B and T cells does not result in demyelination. In apparent contradiction to these results, robust demyelination is detected in MHV-infected young nude (athymic) mice. Herein, we show that demyelination in nude mice was mediated by gamma delta T cells. These cells, but not conventional CD4 or CD8 alpha beta T cells, were detected in the central nervous system of MHV-infected nude mice and their depletion with neutralizing antibody resulted in an 80% reduction in demyelination. These results show, for the first time, that gamma delta T cells can substitute for alpha beta T cells in a virus model of demyelination and further support a pathological role for gamma delta T cells in patients with multiple sclerosis.
Figures



References
-
- Hemmer B, Archelos JJ, Hartung HP: New concepts in the immunopathogenesis of multiple sclerosis. Nat Rev Neurosci 2002, 3:291-301 - PubMed
-
- Noseworthy JH, Lucchinetti C, Rodriguez M, Weinshenker BG: Multiple sclerosis. N Engl J Med 2000, 343:938-952 - PubMed
-
- Tsunoda I, Fujinami RS: Two models of multiple sclerosis: experimental allergic encephalomyelitis and Theiler’s murine encephalomyelitis virus. J Neuropathol Exp Neurol 1996, 55:672-686 - PubMed
-
- Steinman L: Assessment of animal models for MS and demyelinating disease in the design of rational therapy. Neuron 1999, 24:511-514 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

