Osteoprotegerin reduces osteoclast numbers and prevents bone erosion in collagen-induced arthritis
- PMID: 12368214
- PMCID: PMC1867274
- DOI: 10.1016/S0002-9440(10)64417-3
Osteoprotegerin reduces osteoclast numbers and prevents bone erosion in collagen-induced arthritis
Abstract
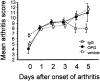
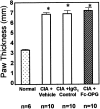
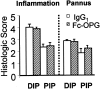
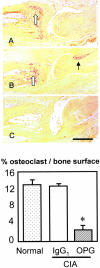
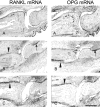
Rheumatoid arthritis is characterized by progressive synovial inflammation and joint destruction. While matrix metalloproteinases (MMPs) are implicated in the erosion of unmineralized cartilage, bone destruction involves osteoclasts, the specialized cells that resorb calcified bone matrix. RANK ligand (RANKL) expressed by stromal cells and T cells, and its cognate receptor, RANK, were identified as a critical ligand-receptor pair for osteoclast differentiation and survival. A decoy receptor for RANKL, osteoprotegerin, (OPG) impinges on this system and regulates osteoclast numbers and activity. RANKL is also expressed in collagen-induced arthritis (CIA) in which focal collections of osteoclasts are prominent at sites of bone destruction. To determine the role of RANK signaling events in the effector phase of CIA, we investigated effects of Fc-osteoprotegerin fusion protein (Fc-OPG) in CIA. After induction of CIA in Dark Agouti rats, test animals were treated with or without Fc-OPG (3 mg/kg/day) subcutaneously for 5 days, beginning at the onset of disease. Paraffin-embedded joints were then analyzed histologically and the adjacent bone assessed by histomorphometry. Osteoclasts were identified using TRAP staining and expression of the mRNA for OPG and RANKL was identified by in situ hybridization. The results indicated that short-term Fc-OPG effectively prevented joint destruction, even though it had no impact on the inflammatory aspects of CIA. In arthritic joints, Fc-OPG depleted osteoclast numbers by over 75% and diminished bone erosion scores by over 60%. Although cartilage loss was also reduced by Fc-OPG, the effects on cartilage were less striking than those on bone. In arthritic joints OPG mRNA was highly expressed and co-localized with RANK ligand, and treatment with Fc-OPG did not affect the expression of endogenous RANKL or OPG mRNA. These data demonstrate that short term Fc-OPG treatment has powerful anti-erosive effects, principally on bone, even though synovitis is not affected. These findings indicate the potential utility of disrupting RANK signaling to preserve skeletal integrity in inflammatory arthritis.
Figures


References
-
- Gough A, Sambrook P, Devlin J, Huissoon A, Njeh C, Robbins S, Nguyen T, Emery P: Osteoclastic activation is the principal mechanism leading to secondary osteoporosis in rheumatoid arthritis. J Rheumatol 1998, 25:1282-1289 - PubMed
-
- Kong YY, Yoshida H, Sarosi I, Tan HL, Timms E, Capparelli C, Morony S, Oliveira-dos-Santos AJ, Van G, Itie A, Khoo W, Wakeham A, Dunstan CR, Lacey DL, Mak TW, Boyle WJ, Penninger JM: OPGL is a key regulator of osteoclastogenesis, lymphocyte development, and lymph-node organogenesis. Nature 1999, 397:315-323 - PubMed
-
- Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR, Burgess T, Elliott R, Colombero A, Elliott G, Scully S, Hsu H, Sullivan J, Hawkins N, Davy E, Capparelli C, Eli A, Qian YX, Kaufman S, Sarosi I, Shalhoub V, Senaldi G, Guo J, Delaney J, Boyle WJ: Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell 1998, 93:165-176 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

