c-Myc is essential for vasculogenesis and angiogenesis during development and tumor progression
- PMID: 12368264
- PMCID: PMC187450
- DOI: 10.1101/gad.1024602
c-Myc is essential for vasculogenesis and angiogenesis during development and tumor progression
Abstract
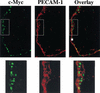
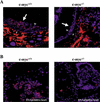
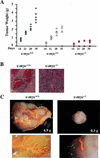
c-Myc promotes cell growth and transformation by ill-defined mechanisms. c-myc(-/-) mice die by embryonic day 10.5 (E10.5) with defects in growth and in cardiac and neural development. Here we report that the lethality of c-myc(-/-) embryos is also associated with profound defects in vasculogenesis and primitive erythropoiesis. Furthermore, c-myc(-/-) embryonic stem (ES) and yolk sac cells are compromised in their differentiative and growth potential. These defects are intrinsic to c-Myc, and are in part associated with a requirement for c-Myc for the expression of vascular endothelial growth factor (VEGF), as VEGF can partially rescue these defects. However, c-Myc is also required for the proper expression of other angiogenic factors in ES and yolk sac cells, including angiopoietin-2, and the angiogenic inhibitors thrombospondin-1 and angiopoietin-1. Finally, c-myc(-/-) ES cells are dramatically impaired in their ability to form tumors in immune-compromised mice, and the small tumors that sometimes develop are poorly vascularized. Therefore, c-Myc function is also necessary for the angiogenic switch that is indispensable for the progression and metastasis of tumors. These findings support the model wherein c-Myc promotes cell growth and transformation, as well as vascular and hematopoietic development, by functioning as a master regulator of angiogenic factors.
Figures





References
-
- Askew DS, Ashmun RA, Simmons BC, Cleveland JL. Constitutive c-myc expression in an IL-3-dependent myeloid cell line suppresses cell cycle arrest and accelerates apoptosis. Oncogene. 1991;6:1915–1922. - PubMed
-
- Barr LF, Campbell SE, Diette GB, Gabrielson EW, Kim S, Shim H, Dang CV. c-Myc suppresses the tumorigenicity of lung cancer cells and down-regulates vascular endothelial growth factor expression. Cancer Res. 2000;60:143–149. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
