Transient induction of cyclin T1 during human macrophage differentiation regulates human immunodeficiency virus type 1 Tat transactivation function
- PMID: 12368300
- PMCID: PMC136632
- DOI: 10.1128/jvi.76.21.10579-10587.2002
Transient induction of cyclin T1 during human macrophage differentiation regulates human immunodeficiency virus type 1 Tat transactivation function
Abstract
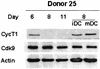
The human immunodeficiency virus type 1 (HIV-1) Tat protein is essential for viral replication and stimulates transcription of the integrated provirus by recruiting the kinase complex TAK/P-TEFb, composed of cyclin T1 (CycT1) and Cdk9, to the viral TAR RNA element. TAK/P-TEFb phosphorylates the RNA polymerase II complex and stimulates transcriptional elongation. In this report, we investigated the regulation of TAK/P-TEFb in primary human macrophages, a major target cell of HIV infection. While Cdk9 levels remained constant, CycT1 protein expression in freshly isolated monocytes was very low, increased early during macrophage differentiation, and, unexpectedly, decreased to very low levels after about 1 week in culture. The kinase activity of TAK/P-TEFb paralleled the changes in CycT1 protein expression. RNA analysis indicated that the transient induction of CycT1 protein expression involves a posttranscriptional mechanism. In transient transfection assays, the ability of Tat to transactivate the HIV long terminal repeat (LTR) in the late differentiated macrophages was greatly diminished relative to its ability to transactivate the HIV LTR in early differentiated cells, strongly suggesting that CycT1 is limiting for Tat function in late differentiated macrophages. Interestingly, lipopolysaccharide, a component of the cell wall of gram-negative bacteria, reinduced CycT1 expression late in macrophage differentiation. These results raise the possibility that regulation of CycT1 expression may be involved in establishing latent infection in macrophages and that opportunistic infection may reactivate the virus by inducing CycT1 expression.
Figures






Similar articles
-
CDK9 autophosphorylation regulates high-affinity binding of the human immunodeficiency virus type 1 tat-P-TEFb complex to TAR RNA.Mol Cell Biol. 2000 Sep;20(18):6958-69. doi: 10.1128/MCB.20.18.6958-6969.2000. Mol Cell Biol. 2000. PMID: 10958691 Free PMC article.
-
Recruitment of cyclin T1/P-TEFb to an HIV type 1 long terminal repeat promoter proximal RNA target is both necessary and sufficient for full activation of transcription.Proc Natl Acad Sci U S A. 1999 Jul 6;96(14):7791-6. doi: 10.1073/pnas.96.14.7791. Proc Natl Acad Sci U S A. 1999. PMID: 10393900 Free PMC article.
-
Regulatory functions of Cdk9 and of cyclin T1 in HIV tat transactivation pathway gene expression.J Cell Biochem. 1999 Dec 1;75(3):357-68. J Cell Biochem. 1999. PMID: 10536359 Review.
-
Regulation of TAK/P-TEFb in CD4+ T lymphocytes and macrophages.Curr HIV Res. 2003 Oct;1(4):395-404. doi: 10.2174/1570162033485159. Curr HIV Res. 2003. PMID: 15049426 Review.
-
Human and rodent transcription elongation factor P-TEFb: interactions with human immunodeficiency virus type 1 tat and carboxy-terminal domain substrate.J Virol. 1999 Jul;73(7):5448-58. doi: 10.1128/JVI.73.7.5448-5458.1999. J Virol. 1999. PMID: 10364292 Free PMC article.
Cited by
-
Cyclin L2 is a critical HIV dependency factor in macrophages that controls SAMHD1 abundance.Cell Host Microbe. 2015 Jan 14;17(1):98-106. doi: 10.1016/j.chom.2014.11.009. Epub 2014 Dec 18. Cell Host Microbe. 2015. PMID: 25532805 Free PMC article.
-
Role of noncoding RNAs in the regulation of P-TEFb availability and enzymatic activity.Biomed Res Int. 2014;2014:643805. doi: 10.1155/2014/643805. Epub 2014 Feb 19. Biomed Res Int. 2014. PMID: 24701579 Free PMC article. Review.
-
Activation of P-TEFb at sites of dual HIV/TB infection, and inhibition of MTB-induced HIV transcriptional activation by the inhibitor of CDK9, Indirubin-3'-monoxime.AIDS Res Hum Retroviruses. 2012 Feb;28(2):182-7. doi: 10.1089/aid.2010.0211. Epub 2011 May 6. AIDS Res Hum Retroviruses. 2012. PMID: 21453127 Free PMC article.
-
miR-198 inhibits HIV-1 gene expression and replication in monocytes and its mechanism of action appears to involve repression of cyclin T1.PLoS Pathog. 2009 Jan;5(1):e1000263. doi: 10.1371/journal.ppat.1000263. Epub 2009 Jan 16. PLoS Pathog. 2009. PMID: 19148268 Free PMC article.
-
Reactivation of Latent HIV-1 Expression by Engineered TALE Transcription Factors.PLoS One. 2016 Mar 2;11(3):e0150037. doi: 10.1371/journal.pone.0150037. eCollection 2016. PLoS One. 2016. PMID: 26933881 Free PMC article.
References
-
- Blankson, J. N., D. Persaud, and R. F. Siliciano. 2002. The challenge of viral reservoirs in HIV-1 infection. Annu. Rev. Med. 53:557-593. - PubMed
-
- Boyum, A. 1968. Isolation of mononuclear cells and granulocytes from human blood. Isolation of mononuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand. J. Clin. Lab. Investig. Suppl. 97:77-89. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

