Prevalence and quantitation of species C adenovirus DNA in human mucosal lymphocytes
- PMID: 12368303
- PMCID: PMC136639
- DOI: 10.1128/jvi.76.21.10608-10616.2002
Prevalence and quantitation of species C adenovirus DNA in human mucosal lymphocytes
Abstract
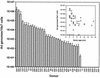
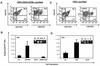
The common species C adenoviruses (serotypes Ad1, Ad2, Ad5, and Ad6) infect more than 80% of the human population early in life. Following primary infection, the virus can establish an asymptomatic persistent infection in which infectious virions are shed in feces for several years. The probable source of persistent virus is mucosa-associated lymphoid tissue, although the molecular details of persistence or latency of adenovirus are currently unknown. In this study, a sensitive real-time PCR assay was developed to quantitate species C adenovirus DNA in human tissues removed for routine tonsillectomy or adenoidectomy. Using this assay, species C DNA was detected in Ficoll-purified lymphocytes from 33 of 42 tissue specimens tested (79%). The levels varied from fewer than 10 to greater than 2 x 10(6) copies of the adenovirus genome/10(7) cells, depending on the donor. DNA from serotypes Ad1, Ad2, and Ad5 was detected, while the rarer serotype Ad6 was not. When analyzed as a function of donor age, the highest levels of adenovirus genomes were found among the youngest donors. Antibody-coated magnetic beads were used to purify lymphocytes into subpopulations and determine whether viral DNA could be enriched within any purified subpopulations. Separation of T cells (CD4/8- expressing and/or CD3-expressing cells) enriched viral DNA in each of nine donors tested. In contrast, B-cell purification (CD19-expressing cells) invariably depleted or eliminated viral DNA. Despite the frequent finding of significant quantities of adenovirus DNA in tonsil and adenoid tissues, infectious virus was rarely present, as measured by coculture with permissive cells. These findings suggest that human mucosal T lymphocytes may harbor species C adenoviruses in a quiescent, perhaps latent form.
Figures



Similar articles
-
Prevalence and quantitation of adenovirus DNA from human tonsil and adenoid tissues.J Med Virol. 2013 Nov;85(11):1947-54. doi: 10.1002/jmv.23678. Epub 2013 Jul 12. J Med Virol. 2013. PMID: 23852770
-
Latent species C adenoviruses in human tonsil tissues.J Virol. 2009 Mar;83(6):2417-28. doi: 10.1128/JVI.02392-08. Epub 2008 Dec 24. J Virol. 2009. PMID: 19109384 Free PMC article.
-
Histone Deacetylase Inhibitors Promote Latent Adenovirus Reactivation from Tonsillectomy Specimens.J Virol. 2020 Jun 1;94(12):e00100-20. doi: 10.1128/JVI.00100-20. Print 2020 Jun 1. J Virol. 2020. PMID: 32269118 Free PMC article.
-
Epigenetics and the dynamics of chromatin during adenovirus infections.FEBS Lett. 2019 Dec;593(24):3551-3570. doi: 10.1002/1873-3468.13697. Epub 2019 Dec 15. FEBS Lett. 2019. PMID: 31769503 Free PMC article. Review.
-
The life and times of adenoviruses.Adv Virus Res. 1999;54:1-13. doi: 10.1016/s0065-3527(08)60363-2. Adv Virus Res. 1999. PMID: 10547672 Review.
Cited by
-
The ubiquity of asymptomatic respiratory viral infections in the tonsils and adenoids of children and their impact on airway obstruction.Int J Pediatr Otorhinolaryngol. 2016 Nov;90:128-132. doi: 10.1016/j.ijporl.2016.09.006. Epub 2016 Sep 14. Int J Pediatr Otorhinolaryngol. 2016. PMID: 27729119 Free PMC article.
-
Presence of adenovirus species C in infiltrating lymphocytes of human sarcoma.PLoS One. 2013 May 6;8(5):e63646. doi: 10.1371/journal.pone.0063646. Print 2013. PLoS One. 2013. PMID: 23671688 Free PMC article.
-
Severe acute respiratory infection in children in a densely populated urban slum in Kenya, 2007-2011.BMC Infect Dis. 2015 Feb 25;15:95. doi: 10.1186/s12879-015-0827-x. BMC Infect Dis. 2015. PMID: 25879805 Free PMC article.
-
Cell-free transmission of human adenovirus by passive mass transfer in cell culture simulated in a computer model.J Virol. 2012 Sep;86(18):10123-37. doi: 10.1128/JVI.01102-12. Epub 2012 Jul 11. J Virol. 2012. PMID: 22787215 Free PMC article.
-
Intestinal HAdV Infection: Tissue Specificity, Persistence, and Implications for Antiviral Therapy.Viruses. 2019 Aug 30;11(9):804. doi: 10.3390/v11090804. Viruses. 2019. PMID: 31480296 Free PMC article. Review.
References
-
- Andiman, W. A., R. I. Jacobson, and G. Tucker. 1977. Leukocyte-associated viremia with adenovirus type 2 in an infant with lower-respiratory-tract disease. N. Engl. J. Med. 297:100-101. - PubMed
-
- Avila, M. M., G. Carballal, H. Rovaletti, B. Ebekian, M. Cusminsky, and M. Weissenbacher. 1989. Viral etiology in acute lower respiratory infections in children from a closed community. Am. Rev. Respir. Dis. 140:634-637. - PubMed
-
- Babcock, G. J., L. L. Decker, M. Volk, and D. A. Thorley-Lawson. 1998. EBV persistence in memory B cells in vivo. Immunity 9:395-404. - PubMed
-
- Brandt, C. D., H. W. Kim, A. J. Vargosko, B. C. Jeffries, J. O. Arrobio, B. Rindge, R. H. Parrott, and R. M. Chanock. 1969. Infections in 18,000 infants and children in a controlled study of respiratory tract disease. I. Adenovirus pathogenicity in relation to serologic type and illness syndrome. Am. J. Epidemiol. 90:484-500. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

