A new type of adenovirus vector that utilizes homologous recombination to achieve tumor-specific replication
- PMID: 12368342
- PMCID: PMC136641
- DOI: 10.1128/jvi.76.21.10994-11002.2002
A new type of adenovirus vector that utilizes homologous recombination to achieve tumor-specific replication
Abstract
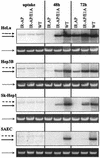
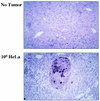
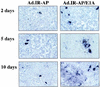
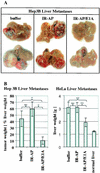
We have developed a new class of adenovirus vectors that selectively replicate in tumor cells. The vector design is based on our recent observation that a variety of human tumor cell lines support DNA replication of adenovirus vectors with deletions of the E1A and E1B genes, whereas primary human cells or mouse liver cells in vivo do not. On the basis of this tumor-selective replication, we developed an adenovirus system that utilizes homologous recombination between inverted repeats to mediate precise rearrangements within the viral genome resulting in replication-dependent activation of transgene expression in tumors (Ad.IR vectors). Here, we used this system to achieve tumor-specific expression of adenoviral wild-type E1A in order to enhance viral DNA replication and spread within tumor metastases. In vitro DNA replication and cytotoxicity studies demonstrated that the mechanism of E1A-enhanced replication of Ad.IR-E1A vectors is efficiently and specifically activated in tumor cells, but not in nontransformed human cells. Systemic application of the Ad.IR-E1A vector into animals with liver metastases achieved transgene expression exclusively in tumors. The number of transgene-expressing tumor cells within metastases increased over time, indicating viral spread. Furthermore, the Ad.IR-E1A vector demonstrated antitumor efficacy in subcutaneous and metastatic models. These new Ad.IR-E1A vectors combine elements that allow for tumor-specific transgene expression, efficient viral replication, and spread in liver metastases after systemic vector application.
Figures





References
-
- Alemany, R., C. Balague, and D. T. Curiel. 2000. Replicative adenoviruses for cancer therapy. Nat. Biotechnol. 18:723-727. - PubMed
-
- Bernt, K. M., D. S. Steinwaerder, S. Ni, Z.-Y. Li, S. R. Roffler, and A. Lieber. 2002. Enzyme-activated prodrug therapy enhances tumor-specific replication of adenovirus vectors. Cancer Res., in press. - PubMed
-
- Bischoff, J. R., D. H. Kirn, A. Williams, C. Heise, S. Horn, M. Muna, L. Ng, J. A. Nye, A. Sampson-Johannes, A. Fattaey, and F. McCormick. 1996. An adenovirus mutant that replicates selectively in p53-deficient human tumor cells. Science 274:373-376. - PubMed
-
- Bruder, J. T., A. Appiah, W. M. Kirkman III, P. Chen, J. Tian, D. Reddy, D. E. Brough, A. Lizonova, and I. Kovesdi. 2000. Improved production of adenovirus vectors expressing apoptotic transgenes. Hum. Gene Ther. 11:139-149. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

