Evolution of phenotypic drug susceptibility and viral replication capacity during long-term virologic failure of protease inhibitor therapy in human immunodeficiency virus-infected adults
- PMID: 12368352
- PMCID: PMC136622
- DOI: 10.1128/jvi.76.21.11104-11112.2002
Evolution of phenotypic drug susceptibility and viral replication capacity during long-term virologic failure of protease inhibitor therapy in human immunodeficiency virus-infected adults
Abstract
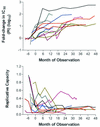
Continued use of antiretroviral therapy despite the emergence of drug-resistant human immunodeficiency virus (HIV) has been associated with the durable maintenance of plasma HIV RNA levels below pretherapy levels. The factors that may account for this partial control of viral replication were assessed in a longitudinal observational study of 20 HIV-infected adults who remained on a stable protease inhibitor-based regimen despite ongoing viral replication (plasma HIV RNA levels consistently >500 copies/ml). Longitudinal plasma samples (n = 248) were assayed for drug susceptibility and viral replication capacity (measured by using a single-cycle recombinant-virus assay). The initial treatment-mediated decrease in plasma viremia was directly proportional to the reduction in replicative capacity (P = 0.01). Early virologic rebound was associated the emergence of a virus population exhibiting increased protease inhibitor phenotypic resistance, while replicative capacity remained low. During long-term virologic failure, plasma HIV RNA levels often remained stable or increased slowly, while phenotypic resistance continued to increase and replicative capacity decreased slowly. The emergence of primary genotypic mutations within protease (particularly V82A, I84V, and L90M) was temporally associated with increasing phenotypic resistance and decreasing replicative capacity, while the emergence of secondary mutations within protease was associated with more-gradual changes in both phenotypic resistance and replicative capacity. We conclude that HIV may be constrained in its ability to become both highly resistant and highly fit and that this may contribute to the continued partial suppression of plasma HIV RNA levels that is observed in some patients with drug-resistant viremia.
Figures

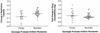

References
-
- Back, N. K., M. Nijhuis, W. Keulen, C. A. Boucher, B. O. Oude Essink, A. B. van Kuilenburg, A. H. van Gennip, and B. Berkhout. 1996. Reduced replication of 3TC-resistant HIV-1 variants in primary cells due to a processivity defect of the reverse transcriptase enzyme. EMBO J. 15:4040-4049. - PMC - PubMed
-
- Belec, L., C. Piketty, A. Si-Mohamed, C. Goujon, M. C. Hallouin, S. Cotigny, L. Weiss, and M. D. Kazatchkine. 2000. High levels of drug-resistant human immunodeficiency virus variants in patients exhibiting increasing CD4+ T cell counts despite virologic failure of protease inhibitor-containing antiretroviral combination therapy. J. Infect. Dis. 181:1808-1812. - PubMed
-
- Berkhout, B. 1999. HIV-1 evolution under pressure of protease inhibitors: climbing the stairs of viral fitness. J. Biomed. Sci. 6:298-305. - PubMed
-
- Bleiber, G., M. Munoz, A. Ciuffi, P. Meylan, and A. Telenti. 2001. Individual contributions of mutant protease and reverse transcriptase to viral infectivity, replication, and protein maturation of antiretroviral drug-resistant human immunodeficiency virus type 1. J. Virol. 75:3291-3300. - PMC - PubMed
-
- Borman, A. M., S. Paulous, and F. Clavel. 1996. Resistance of human immunodeficiency virus type 1 to protease inhibitors: selection of resistance mutations in the presence and absence of the drug. J. Gen. Virol. 77:419-426. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

