Implication of the lymphocyte-specific nuclear body protein Sp140 in an innate response to human immunodeficiency virus type 1
- PMID: 12368356
- PMCID: PMC136615
- DOI: 10.1128/jvi.76.21.11133-11138.2002
Implication of the lymphocyte-specific nuclear body protein Sp140 in an innate response to human immunodeficiency virus type 1
Abstract
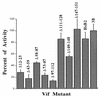
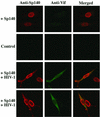
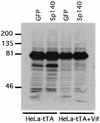
The viral infectivity factor (Vif) of human immunodeficiency virus type 1 (HIV-1) neutralizes an unidentified antiviral pathway that occurs only in nonpermissive (NP) cells. Using a yeast two-hybrid screen of a human lymphocyte cDNA library, we identified several potential Vif partners. One, the nuclear body protein Sp140, was found specifically in all NP cells (n = 12 cell lines tested; P < or = 0.001), and HIV-1 infection induced its partial dispersal from nuclear bodies into cytosolic colocalization with Vif. Our results implicate Sp140 in a response to HIV-1 that may be related to or coordinated with the pathway that inactivates HIV-1 lacking vif.
Figures
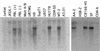

Similar articles
-
Cellular and viral specificities of human immunodeficiency virus type 1 vif protein.J Virol. 2000 Jul;74(13):5982-7. doi: 10.1128/jvi.74.13.5982-5987.2000. J Virol. 2000. PMID: 10846079 Free PMC article.
-
Human immunodeficiency virus type 1 Vif does not influence expression or virion incorporation of gag-, pol-, and env-encoded proteins.J Virol. 1996 Dec;70(12):8263-9. doi: 10.1128/JVI.70.12.8263-8269.1996. J Virol. 1996. PMID: 8970945 Free PMC article.
-
Human immunodeficiency virus type 1 Vif protein is an integral component of an mRNP complex of viral RNA and could be involved in the viral RNA folding and packaging process.J Virol. 2000 Sep;74(18):8252-61. doi: 10.1128/jvi.74.18.8252-8261.2000. J Virol. 2000. PMID: 10954522 Free PMC article.
-
The Vif protein of human immunodeficiency virus type 1 (HIV-1): enigmas and solutions.Curr Med Chem. 2004 Jan;11(2):221-31. doi: 10.2174/0929867043456124. Curr Med Chem. 2004. PMID: 14754418 Review.
-
The viral infectivity factor (Vif) of HIV-1 unveiled.Trends Mol Med. 2004 Jun;10(6):291-7. doi: 10.1016/j.molmed.2004.04.008. Trends Mol Med. 2004. PMID: 15177194 Review.
Cited by
-
Post-GWAS functional characterization of susceptibility variants for chronic lymphocytic leukemia.PLoS One. 2012;7(1):e29632. doi: 10.1371/journal.pone.0029632. Epub 2012 Jan 3. PLoS One. 2012. PMID: 22235315 Free PMC article.
-
Immune chromatin reader SP140 regulates microbiota and risk for inflammatory bowel disease.Cell Host Microbe. 2022 Oct 12;30(10):1370-1381.e5. doi: 10.1016/j.chom.2022.08.018. Epub 2022 Sep 20. Cell Host Microbe. 2022. PMID: 36130593 Free PMC article.
-
SP140L, an Evolutionarily Recent Member of the SP100 Family, Is an Autoantigen in Primary Biliary Cirrhosis.J Immunol Res. 2015;2015:526518. doi: 10.1155/2015/526518. Epub 2015 Aug 11. J Immunol Res. 2015. PMID: 26347895 Free PMC article.
-
The human immunodeficiency virus type 1 Vif protein reduces intracellular expression and inhibits packaging of APOBEC3G (CEM15), a cellular inhibitor of virus infectivity.J Virol. 2003 Nov;77(21):11398-407. doi: 10.1128/jvi.77.21.11398-11407.2003. J Virol. 2003. PMID: 14557625 Free PMC article.
-
The Speckled Protein (SP) Family: Immunity's Chromatin Readers.Trends Immunol. 2020 Jul;41(7):572-585. doi: 10.1016/j.it.2020.04.007. Epub 2020 May 5. Trends Immunol. 2020. PMID: 32386862 Free PMC article. Review.
References
-
- Bernier-Villamor, V., D. A. Sampson, M. J. Matunis, and C. D. Lima. 2002. Structural basis for E2-mediated SUMO conjugation revealed by a complex between ubiquitin-conjugating enzyme Ubc9 and RanGAP1. Cell 108:345-356. - PubMed
-
- Bloch, D. B., S. M. de la Monte, P. Guigaouri, A. Filippov, and K. D. Bloch. 1996. Identification and characterization of a leukocyte-specific component of the nuclear body. J. Biol. Chem. 271:29198-29204. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

