A phosphate transporter from Medicago truncatula involved in the acquisition of phosphate released by arbuscular mycorrhizal fungi
- PMID: 12368495
- PMCID: PMC151226
- DOI: 10.1105/tpc.004861
A phosphate transporter from Medicago truncatula involved in the acquisition of phosphate released by arbuscular mycorrhizal fungi
Abstract
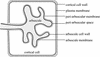
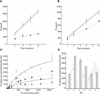
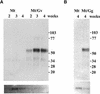
Many plants have the capacity to obtain phosphate via a symbiotic association with arbuscular mycorrhizal (AM) fungi. In AM associations, the fungi release phosphate from differentiated hyphae called arbuscules, that develop within the cortical cells, and the plant transports the phosphate across a symbiotic membrane, called the periarbuscular membrane, into the cortical cell. In Medicago truncatula, a model legume used widely for studies of root symbioses, it is apparent that the phosphate transporters known to operate at the root-soil interface do not participate in symbiotic phosphate transport. EST database searches with short sequence motifs shared by known phosphate transporters enabled the identification of a novel phosphate transporter from M. truncatula, MtPT4. MtPT4 is significantly different from the plant root phosphate transporters cloned to date. Complementation of yeast phosphate transport mutants indicated that MtPT4 functions as a phosphate transporter, and estimates of the K(m) suggest a relatively low affinity for phosphate. MtPT4 is expressed only in mycorrhizal roots, and the MtPT4 promoter directs expression exclusively in cells containing arbuscules. MtPT4 is located in the membrane fraction of mycorrhizal roots, and immunolocalization revealed that MtPT4 colocalizes with the arbuscules, consistent with a location on the periarbuscular membrane. The transport properties and spatial expression patterns of MtPT4 are consistent with a role in the acquisition of phosphate released by the fungus in the AM symbiosis.
Figures


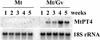


Similar articles
-
Medicago truncatula mtpt4 mutants reveal a role for nitrogen in the regulation of arbuscule degeneration in arbuscular mycorrhizal symbiosis.Plant J. 2011 Dec;68(6):954-65. doi: 10.1111/j.1365-313X.2011.04746.x. Epub 2011 Oct 17. Plant J. 2011. PMID: 21848683
-
Polar localization of a symbiosis-specific phosphate transporter is mediated by a transient reorientation of secretion.Proc Natl Acad Sci U S A. 2012 Mar 13;109(11):E665-72. doi: 10.1073/pnas.1110215109. Epub 2012 Feb 21. Proc Natl Acad Sci U S A. 2012. PMID: 22355114 Free PMC article.
-
The phosphate transporters LjPT4 and MtPT4 mediate early root responses to phosphate status in non mycorrhizal roots.Plant Cell Environ. 2016 Mar;39(3):660-71. doi: 10.1111/pce.12659. Epub 2016 Jan 12. Plant Cell Environ. 2016. PMID: 26476189
-
Cellular programs for arbuscular mycorrhizal symbiosis.Curr Opin Plant Biol. 2012 Dec;15(6):691-8. doi: 10.1016/j.pbi.2012.08.010. Epub 2012 Oct 1. Curr Opin Plant Biol. 2012. PMID: 23036821 Review.
-
Arbuscular mycorrhizal symbiosis and plant aquaporin expression.Phytochemistry. 2007 Jan;68(1):122-9. doi: 10.1016/j.phytochem.2006.09.033. Epub 2006 Nov 15. Phytochemistry. 2007. PMID: 17109903 Review.
Cited by
-
The pattern of Phosphate transporter 1 genes evolutionary divergence in Glycine max L.BMC Plant Biol. 2013 Mar 20;13:48. doi: 10.1186/1471-2229-13-48. BMC Plant Biol. 2013. PMID: 23510338 Free PMC article.
-
Diversity of morphology and function in arbuscular mycorrhizal symbioses in Brachypodium distachyon.Planta. 2012 Sep;236(3):851-65. doi: 10.1007/s00425-012-1677-z. Epub 2012 Jun 19. Planta. 2012. PMID: 22711284
-
Arbuscular mycorrhizal fungal communities and Rhizophagus irregularis populations shift in response to short-term ploughing and fertilisation in a buffer strip.Mycorrhiza. 2016 Jan;26(1):33-46. doi: 10.1007/s00572-015-0644-5. Epub 2015 May 29. Mycorrhiza. 2016. PMID: 26023005
-
Two putative-aquaporin genes are differentially expressed during arbuscular mycorrhizal symbiosis in Lotus japonicus.BMC Plant Biol. 2012 Oct 9;12:186. doi: 10.1186/1471-2229-12-186. BMC Plant Biol. 2012. PMID: 23046713 Free PMC article.
-
Arbuscular Mycorrhizal Fungi Confer Salt Tolerance in Giant Reed (Arundo donax L.) Plants Grown Under Low Phosphorus by Reducing Leaf Na+ Concentration and Improving Phosphorus Use Efficiency.Front Plant Sci. 2019 Jul 16;10:843. doi: 10.3389/fpls.2019.00843. eCollection 2019. Front Plant Sci. 2019. PMID: 31396243 Free PMC article.
References
-
- Alexander, T., Meier, R., Toth, R., and Weber, H.C. (1988). Dynamics of arbuscule development and degeneration in mycorrhizas of Triticum aestivum L. and Avena sativa L. with reference to Zea mays L. New Phytol. 110, 363–370.
-
- Alexander, T., Toth, R., Meier, R., and Weber, H.C. (1989). Dynamics of arbuscule development and degeneration in onion, bean and tomato with reference to vesicular-arbuscular mycorrhizae in grasses. Can. J. Bot. 67, 2505–2513.
-
- Arnon, D.I., and Hoagland, D.R. (1940). Crop production in artificial culture solutions and in soils with special reference to factors influencing yields and absorption of inorganic nutrients. Soil Sci. 50, 463–483.
-
- Bailey, T.L., and Elkan, C. (1994). Fitting a mixture model by expectation maximization to discover motifs in biopolymers. In Proceedings of the Second International Conference on Intelligent Systems for Molecular Biology. (Menlo Park, CA: AAAI Press), pp. 28–36. - PubMed
-
- Barker, D.G., et al. (1990). Medicago truncatula, a model plant for studying the molecular genetics of the Rhizobium-legume symbiosis. Plant Mol. Biol. Rep. 8, 40–49.
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

