QUASIMODO1 encodes a putative membrane-bound glycosyltransferase required for normal pectin synthesis and cell adhesion in Arabidopsis
- PMID: 12368506
- PMCID: PMC151237
- DOI: 10.1105/tpc.004259
QUASIMODO1 encodes a putative membrane-bound glycosyltransferase required for normal pectin synthesis and cell adhesion in Arabidopsis
Abstract
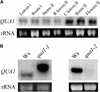
Pectins are a highly complex family of cell wall polysaccharides. As a result of a lack of specific mutants, it has been difficult to study the biosynthesis of pectins and their role in vivo. We have isolated two allelic mutants, named quasimodo1 (qua1-1 and qua1-2), that are dwarfed and show reduced cell adhesion. Mutant cell walls showed a 25% reduction in galacturonic acid levels compared with the wild type, indicating reduced pectin content, whereas neutral sugars remained unchanged. Immersion immunofluorescence with the JIM5 and JIM7 monoclonal antibodies that recognize homogalacturonan epitopes revealed less labeling of mutant roots compared with the wild type. Both mutants carry a T-DNA insertion in a gene (QUA1) that encodes a putative membrane-bound glycosyltransferase of family 8. We present evidence for the possible involvement of a glycosyltransferase of this family in the synthesis of pectic polysaccharides, suggesting that other members of this large multigene family in Arabidopsis also may be important for pectin biosynthesis. The mutant phenotype is consistent with a central role for pectins in cell adhesion.
Figures







References
-
- Altschul, S.F., Gish, W., Miller, W., Myers, E.W., and Lipman, D.J. (1990). Basic local alignment search tool. J. Mol. Biol. 215, 403–410. - PubMed
-
- Benen, J.A.E., Kester, H.C.M., and Visser, J. (1999). Kinetic characterization of Aspergillus niger N400 endopolygalacturonases I, II and C. Eur. J. Biochem. 259, 577–585. - PubMed
-
- Bouchez, D., Camilleri, C., and Caboche, M. (1993). A binary vector based on Basta resistance for in planta transformation of Arabidopsis thaliana. C. R. Acad. Sci. Paris 316, 1188–1193.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

