Antihypertensive efficacy of olmesartan medoxomil, a new angiotensin II receptor antagonist, as assessed by ambulatory blood pressure measurements
- PMID: 12368570
- PMCID: PMC8101835
- DOI: 10.1111/j.1524-6175.2002.01051.x
Antihypertensive efficacy of olmesartan medoxomil, a new angiotensin II receptor antagonist, as assessed by ambulatory blood pressure measurements
Abstract
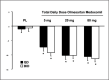
Olmesartan medoxomil is a new angiotensin II receptor blocker. In this randomized, double-blind, placebo-controlled study, the efficacy and safety of olmesartan medoxomil was assessed in 334 patients with moderate to severe essential hypertension. Patients were randomized to receive placebo; 5, 20, or 80 mg olmesartan medoxomil q.d.; or 2.5, 10, or 40 mg olmesartan medoxomil b.i.d. Ambulatory and cuff blood pressure were measured prior to and after 8 weeks of treatment. Treatment with olmesartan medoxomil resulted in a significant placebo-adjusted reduction of mean 24-hour ambulatory diastolic blood pressure of 9.6 mm Hg, 12.2 mm Hg, and 10.6 mm Hg in the 5-, 20-, and 80-mg q.d. groups, respectively. Corresponding reductions in mean ambulatory systolic blood pressure were 14.5 mm Hg, 16.5 mm Hg, and 15.4 mm Hg. Similar reductions of diastolic and systolic blood pressure were seen with b.i.d. dosing. The diastolic trough-to-peak ratios of the q.d. doses of olmesartan medoxomil ranged from 57%-70%, indicating 24-hour effectiveness. The safety profile of olmesartan medoxomil was similar to that of placebo. Olmesartan medoxomil appears to be a safe and effective once-a-day treatment for hypertension.
Copyright 2002 Le Jacq Communications, Inc.
Figures


References
-
- Burnier M, Brunner HR. Angiotensin II receptor antagonists. Lancet. 2000;355:637–645. - PubMed
-
- Yanagisawa H, Amemiya Y, Kanazaki T, et al. Nonpeptide angiotensin II receptor antagonists: synthesis, biological activities, and structure—activity relationships of imidazole‐5‐carboxyliv acids bearing alkyl, alkenyl, and hydroxyalkyl substituents at the 4‐posotion and their related compounds. J Med Chem. 1996;39:323–338. - PubMed
-
- Anonymous . Olmesartan medoxomil. Drugs Future. 1997;22:1205–1209.
-
- Mizuno M, Sada T, Ikeda M, et al. Pharmacology of olmesartan medoxomil, a novel nonpeptide angiotensin II receptor antagonist. Eur J Pharmacol. 1995;285:181–188. - PubMed
-
- Püchler K, Nussberger J, Laeis P, et al. Blood pressure and endocrine effects of single doses of olmesartan medoxomil, a novel angiotensin II antagonist, in salt‐restricted hypertensive patients. J Hypertens. 1997;15:1809–1812. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

