Prolonged survival of patients receiving active immunotherapy with Canvaxin therapeutic polyvalent vaccine after complete resection of melanoma metastatic to regional lymph nodes
- PMID: 12368672
- PMCID: PMC1422598
- DOI: 10.1097/00000658-200210000-00006
Prolonged survival of patients receiving active immunotherapy with Canvaxin therapeutic polyvalent vaccine after complete resection of melanoma metastatic to regional lymph nodes
Abstract
Objective: To determine whether adjuvant postoperative active specific immunotherapy with a therapeutic polyvalent vaccine (PV) called Canvaxin can prolong survival following complete resection of melanoma metastatic to regional nodes (American Joint Committee on Cancer [AJCC] stage III melanoma).
Summary background data: Despite complete lymphadenectomy, 5-year overall survival (OS) for patients with melanoma metastatic to regional lymph nodes is only 20% to 50%, depending on the number of tumor-involved nodes. In 1984, the authors began phase II trials of Canvaxin PV as postsurgical adjuvant therapy for AJCC stage III melanoma.
Methods: Patients who received PV between 1984 and 1998 were compared with patients who did not receive PV postsurgical therapy between 1971 and 1998. The seven covariates recently defined by the AJCC Melanoma Staging Committee (number of metastatic nodes, palpable status, ulceration, age, primary site, pT stage, and gender) were included by Cox regression in a multivariate model of OS. A computerized program matched PV and non-PV patients by these covariates.
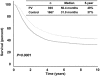
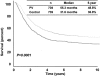
Results: Of 2,602 patients who underwent complete lymphadenectomy for AJCC stage III melanoma with regional nodal metastases and were followed up by the same team of oncologists between 1971 and 1998, 935 received PV and 1,667 did not. Median OS and 5-year OS were significantly higher in PV than non-PV patients (56.4 vs. 31.9 months and 49% vs. 37%, respectively; P =.0001). When the non-PV patients were matched by the four most significant covariates, 447 matched pairs were formed between patients seen before or after January 1, 1985, and the OS was not different between the two time periods ( P=.789). However, when the PV patients were matched with non-PV patients by six covariates forming 739 pairs, the PV patients survived longer ( P=.0001). Detailed analysis of the 1,505 patients who were seen or who began vaccine therapy within 4 months after lymphadenectomy, and who had more complete data on the seven prognostic covariates showed that median OS and 5-year OS were higher in 445 PV patients than in 1,060 non-PV patients: 70.4 versus 31 months and 52% versus 37%, respectively (P =.0001). Multivariate Cox regression analysis identified six significant prognostic factors: number of metastatic nodes, size of metastatic nodes, pT stage, ulceration, age, and PV therapy. PV therapy reduced the relative risk of death to 0.64 (95% confidence interval, 0.55-0.76) ( P=.0001); sex and site of primary were of borderline significance.
Conclusions: This large single-institution study independently confirmed the significance of prognostic covariates in the new AJCC staging system. By using modern statistical methods that controlled for all known prognostic factors, it also demonstrated PV's ability to significantly enhance OS. A multicenter phase III randomized trial is underway to validate the efficacy of PV as a postsurgical adjuvant.
Figures


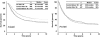
Similar articles
-
Immune response to postsurgical adjuvant active immunotherapy with Canvaxin polyvalent cancer vaccine: correlations with clinical course of patients with metastatic melanoma.Dev Biol (Basel). 2004;116:209-17; discussion 229-36. Dev Biol (Basel). 2004. PMID: 15603194 Clinical Trial.
-
Prolonged survival after complete resection of disseminated melanoma and active immunotherapy with a therapeutic cancer vaccine.J Clin Oncol. 2002 Dec 1;20(23):4549-54. doi: 10.1200/JCO.2002.01.151. J Clin Oncol. 2002. PMID: 12454111 Clinical Trial.
-
TA90 immune complex predicts survival following surgery and adjuvant vaccine immunotherapy for stage IV melanoma.Cancer J Sci Am. 1997 Nov-Dec;3(6):364-70. Cancer J Sci Am. 1997. PMID: 9403050 Clinical Trial.
-
The role of adjuvant therapy in melanoma management.Cancer. 1995 Jan 15;75(2 Suppl):726-34. doi: 10.1002/1097-0142(19950115)75:2+<726::aid-cncr2820751417>3.0.co;2-r. Cancer. 1995. PMID: 7805001 Review.
-
Overview of resistance to systemic therapy in patients with breast cancer.Adv Exp Med Biol. 2007;608:1-22. doi: 10.1007/978-0-387-74039-3_1. Adv Exp Med Biol. 2007. PMID: 17993229 Review.
Cited by
-
BCG turns 100: its nontraditional uses against viruses, cancer, and immunologic diseases.J Clin Invest. 2021 Jun 1;131(11):e148291. doi: 10.1172/JCI148291. J Clin Invest. 2021. PMID: 34060492 Free PMC article. Review.
-
Anal melanoma.Clin Colon Rectal Surg. 2006 May;19(2):78-87. doi: 10.1055/s-2006-942348. Clin Colon Rectal Surg. 2006. PMID: 20011314 Free PMC article.
-
Biology of human cutaneous melanoma.Cancers (Basel). 2010 Mar 12;2(1):165-89. doi: 10.3390/cancers2010165. Cancers (Basel). 2010. PMID: 24281039 Free PMC article.
-
Lymphatic mapping and sentinel lymphadenectomy for early-stage melanoma: therapeutic utility and implications of nodal microanatomy and molecular staging for improving the accuracy of detection of nodal micrometastases.Ann Surg. 2003 Oct;238(4):538-49; discussion 549-50. doi: 10.1097/01.sla.0000086543.45557.cb. Ann Surg. 2003. PMID: 14530725 Free PMC article.
-
Sex-biased adaptive immune regulation in cancer development and therapy.iScience. 2022 Jul 4;25(8):104717. doi: 10.1016/j.isci.2022.104717. eCollection 2022 Aug 19. iScience. 2022. PMID: 35880048 Free PMC article. Review.
References
-
- Jemal A, Thomas A, Murray T, et al. Cancer statistics. CA Cancer J Clin 2002; 52: 23–47. - PubMed
-
- Morton DL, Barth A. Vaccine therapy for malignant melanoma. CA Cancer J Clin 1996; 46: 225–244. - PubMed
-
- Barth A, Morton DL. Role of adjuvant therapy in melanoma management. Cancer 1995; 75: 726–734. - PubMed
-
- Kirkwood JM, Ibrahim JG, Sondak VK, et al. High- and low-dose interferon alfa-2b in high-risk melanoma: first analysis of intergroup trial E1690/S9111/C9190. J Clin Oncol 2000; 18: 2444–2458. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

