Structural organization of the endoplasmic reticulum
- PMID: 12370207
- PMCID: PMC1307613
- DOI: 10.1093/embo-reports/kvf202
Structural organization of the endoplasmic reticulum
Abstract
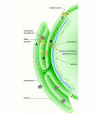
The endoplasmic reticulum (ER) is a continuous membrane system but consists of various domains that perform different functions. Structurally distinct domains of this organelle include the nuclear envelope (NE), the rough and smooth ER, and the regions that contact other organelles. The establishment of these domains and the targeting of proteins to them are understood to varying degrees. Despite its complexity, the ER is a dynamic structure. In mitosis it must be divided between daughter cells and domains must be re-established, and even in interphase it is constantly rearranged as tubules extend along the cytoskeleton. Throughout these rearrangements the ER maintains its basic structure. How this is accomplished remains mysterious, but some insight has been gained from in vitro systems.
Figures




References
-
- Amar-Cortesec A., Dublet B. and Beaufay H. (1989) Translocation and proteolytic processing of nascent secretory polypeptide chains: two functions associated with the ribosomal domain of the endoplasmic reticulum. Mol. Biol. Cell, 65, 99–108. - PubMed
-
- Banta M., Polizotto R.S., Wood S.A., de Figueiredo P. and Brown W.J. (1995) Characterization of a cytosolic activity that induces the formation of Golgi membrane tubules in a cell-free reconstitution system. Biochemistry, 34, 13359–13366. - PubMed
-
- Baumann O. and Walz B. (2001) Endoplasmic reticulum of animal cells and its organization into structural and functional domains. Int. Rev. Cytol., 205, 149–214. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

