The LIM-only protein FHL2 interacts with beta-catenin and promotes differentiation of mouse myoblasts
- PMID: 12370240
- PMCID: PMC2173499
- DOI: 10.1083/jcb.200202075
The LIM-only protein FHL2 interacts with beta-catenin and promotes differentiation of mouse myoblasts
Abstract
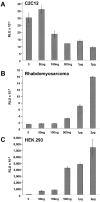
FHL2 is a LIM-domain protein expressed in myoblasts but down-regulated in malignant rhabdomyosarcoma cells, suggesting an important role of FHL2 in muscle development. To investigate the importance of FHL2 during myoblast differentiation, we performed a yeast two-hybrid screen using a cDNA library derived from myoblasts induced for differentiation. We identified beta-catenin as a novel interaction partner of FHL2 and confirmed the specificity of association by direct in vitro binding tests and coimmunoprecipitation assays from cell lysates. Deletion analysis of both proteins revealed that the NH2-terminal part of beta-catenin is sufficient for binding in yeast, but addition of the first armadillo repeat is necessary for binding FHL2 in mammalian cells, whereas the presence of all four LIM domains of FHL2 is needed for the interaction. Expression of FHL2 counteracts beta-catenin-mediated activation of a TCF/LEF-dependent reporter gene in a dose-dependent and muscle cell-specific manner. After injection into Xenopus embryos, FHL2 inhibited the beta-catenin-induced axis duplication. C2C12 mouse myoblasts stably expressing FHL2 show increased myogenic differentiation reflected by accelerated myotube formation and expression of muscle-specific proteins. These data imply that FHL2 is a muscle-specific repressor of LEF/TCF target genes and promotes myogenic differentiation by interacting with beta-catenin.
Figures








References
-
- Akiyama, T. 2000. Wnt/β-catenin signaling. Cytokine Growth Factor Rev. 11:273–282. - PubMed
-
- Beckerle, M.C. 1997. Zyxin: zinc fingers at sites of cell adhesion. Bioessays. 19:949–957. - PubMed
-
- Behrens, J., J.P. von Kries, M. Kuhl, L. Bruhn, D. Wedlich, R. Grosschedl, and W. Birchmeier. 1996. Functional interaction of β-catenin with the transcription factor LEF-1. Nature. 382:638–642. - PubMed

