Interleukin 21 is a T helper (Th) cell 2 cytokine that specifically inhibits the differentiation of naive Th cells into interferon gamma-producing Th1 cells
- PMID: 12370258
- PMCID: PMC2194031
- DOI: 10.1084/jem.20020620
Interleukin 21 is a T helper (Th) cell 2 cytokine that specifically inhibits the differentiation of naive Th cells into interferon gamma-producing Th1 cells
Abstract
The cytokine potential of developing T helper (Th) cells is directly shaped both positively and negatively by the cytokines expressed by the effector Th cell subsets. Here we find that the recently identified cytokine, interleukin (IL)-21, is preferentially expressed by Th2 cells when compared with Th1 cells generated in vitro and in vivo. Exposure of naive Th precursors to IL-21 inhibits interferon (IFN)-gamma production from developing Th1 cells. The repression of IFN-gamma production is specific in that the expression of other Th1 and Th2 cytokines is unaffected. IL-21 decreases the IL-12 responsiveness of developing Th cells by specifically reducing both signal transducer and activator of transcription 4 protein and mRNA expression. These results suggest that Th2 cell-derived IL-21 regulates the development of IFN-gamma-producing Th1 cells which could serve to amplify a Th2 response.
Figures
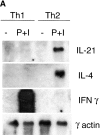





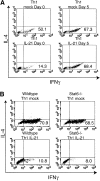

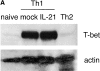



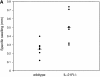

References
-
- Abbas, A.K., K.M. Murphy, and A. Sher. 1996. Functional diversity of helper T lymphocytes. Nature. 383:787–793. - PubMed
-
- Glimcher, L.H., and K.M. Murphy. 2000. Lineage commitment in the immune system: the T helper lymphocyte grows up. Genes Dev. 14:1693–1711. - PubMed
-
- O'Garra, A. 1998. Cytokines induce the development of functionally heterogeneous T helper cell subsets. Immunity. 8:275–283. - PubMed
-
- Maggi, E., P. Parronchi, R. Manetti, C. Simonelli, M.P. Piccinni, F.S. Rugiu, M. De Carli, M. Ricci, and S. Romagnani. 1992. Reciprocal regulatory effects of IFN-γ and IL-4 on the in vitro development of human Th1 and Th2 clones. J. Immunol. 148:2142–2147. - PubMed
-
- Moore, K.W., R. de Waal Malefyt, R.L. Coffman, and A. O'Garra. 2001. Interleukin-10 and the interleukin-10 receptor. Annu. Rev. Immunol. 19:683–765. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

