Inhibition of endothelial cell survival and angiogenesis by protein kinase A
- PMID: 12370271
- PMCID: PMC151143
- DOI: 10.1172/JCI14268
Inhibition of endothelial cell survival and angiogenesis by protein kinase A
Abstract
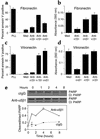
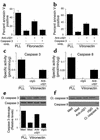
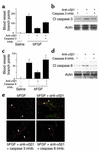
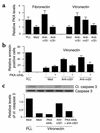
Receptors for the provisional ECM are important regulators of angiogenesis. One of these receptors, integrin alpha5beta1, plays a critical role in tumor- and growth factor-induced angiogenesis, because antagonists of this integrin potently inhibit angiogenesis and tumor growth. Here we show that the integrin alpha5beta1 promotes endothelial cell survival during angiogenesis in vivo by suppressing the activity of protein kinase A (PKA). Antagonists of integrin alpha5beta1 activate PKA, which then leads to the activation of caspase-8 and induction of apoptosis. Direct activation of PKA by cAMP or by expression of the PKA catalytic subunit also induces endothelial cell apoptosis, resulting in angiogenesis inhibition in vivo. Our studies indicate that ligation of integrin alpha5beta1 during angiogenesis suppresses an apoptotic program that is dependent on PKA. These studies also indicate that induction of endothelial cell apoptosis in vivo by genetic or pharmacological activation of PKA may be a useful strategy to inhibit angiogenesis.
Figures




Comment in
-
Endothelial integrins and angiogenesis: not so simple anymore.J Clin Invest. 2002 Oct;110(7):913-4. doi: 10.1172/JCI16713. J Clin Invest. 2002. PMID: 12370267 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

