Targeted disruption of NFATc3, but not NFATc4, reveals an intrinsic defect in calcineurin-mediated cardiac hypertrophic growth
- PMID: 12370307
- PMCID: PMC135666
- DOI: 10.1128/MCB.22.21.7603-7613.2002
Targeted disruption of NFATc3, but not NFATc4, reveals an intrinsic defect in calcineurin-mediated cardiac hypertrophic growth
Abstract
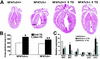
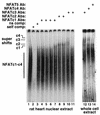
A calcineurin-nuclear factor of activated T cells (NFAT) regulatory pathway has been implicated in the control of cardiac hypertrophy, suggesting one mechanism whereby alterations in intracellular calcium handling are linked to the expression of hypertrophy-associated genes. Although recent studies have demonstrated a necessary role for calcineurin as a mediator of cardiac hypertrophy, the potential involvement of NFAT transcription factors as downstream effectors of calcineurin signaling has not been evaluated. Accordingly, mice with targeted disruptions in NFATc3 and NFATc4 genes were characterized. Whereas the loss of NFATc4 did not compromise the ability of the myocardium to undergo hypertrophic growth, NFATc3-null mice demonstrated a significant reduction in calcineurin transgene-induced cardiac hypertrophy at 19 days, 26 days, 6 weeks, 8 weeks, and 10 weeks of age. NFATc3-null mice also demonstrated attenuated pressure overload- and angiotensin II-induced cardiac hypertrophy. These results provide genetic evidence that calcineurin-regulated responses require NFAT effectors in vivo.
Figures







References
-
- Beals, C. R., C. M. Sheridan, C. W. Turck, P. Gardner, and G. R. Crabtree. 1997. Nuclear export of NF-ATc enhanced by glycogen synthase kinase-3. Science 275:1930-1934. - PubMed
-
- Blaeser, F., H. Ho, R. Prywes, and T. A. Chatila. 2000. Ca2+-dependent gene expression mediated by MEF2 transcription factors. J. Biol. Chem. 275:197-209. - PubMed
-
- Crabtree, G. R. 1999. Generic signals and specific outcomes: signaling through Ca2+, calcineurin, and NF-AT. Cell 96:611-614. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
