Intralysosomal cystine accumulation in mice lacking cystinosin, the protein defective in cystinosis
- PMID: 12370309
- PMCID: PMC135682
- DOI: 10.1128/MCB.22.21.7622-7632.2002
Intralysosomal cystine accumulation in mice lacking cystinosin, the protein defective in cystinosis
Abstract
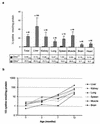
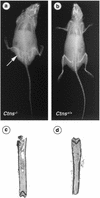
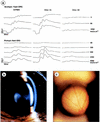
Cystinosis is an autosomal recessive disorder characterized by an accumulation of intralysosomal cystine. The causative gene, CTNS, encodes cystinosin, a seven-transmembrane-domain protein, which we recently showed to be a lysosomal cystine transporter. The most severe and frequent form of cystinosis, the infantile form, appears around 6 to 12 months, with a proximal tubulopathy (de Toni-Debré-Fanconi syndrome) and ocular damage. End-stage renal failure is reached by 10 years of age. Accumulation of cystine in all tissues eventually leads to multisystemic disease. Treatment with cysteamine, which reduces the concentration of intracellular cystine, delays disease progression but has undesirable side effects. We report the first Ctns knockout mouse model generated using a promoter trap approach. We replaced the last four Ctns exons by an internal ribosome entry site-betagal-neo cassette and showed that the truncated protein was mislocalized and nonfunctional. Ctns(-/-) mice accumulated cystine in all organs tested, and cystine crystals, pathognomonic of cystinosis, were observed. Ctns(-/-) mice developed ocular changes similar to those observed in affected individuals, bone defects and behavioral anomalies. Interestingly, Ctns(-/-) mice did not develop signs of a proximal tubulopathy, or renal failure. A preliminary therapeutic trial using an oral administration of cysteamine was carried out and demonstrated the efficiency of this treatment for cystine clearance in Ctns(-/-) mice. This animal model will prove an invaluable and unique tool for testing emerging therapeutics for cystinosis.
Figures



References
-
- Anikster, Y., C. Lucero, J. Guo, M. Huizing, V. Shotelersuk, I. Bernardini, G. McDowell, F. Iwata, M. I. Kaiser-Kupfer, R. Jaffe, J. Thoene, J. A. Schneider, and W. A. Gahl. 2000. Ocular nonnephropathic cystinosis: clinical, biochemical, and molecular correlations. Pediatr. Res. 47:17-23. - PubMed
-
- Attard, M., G. Jean, L. Forestier, S. Cherqui, W. van't Hoff, M. Broyer, C. Antignac, and M. Town. 1999. Severity of phenotype in cystinosis varies with mutations in the CTNS gene: predicted effect on the model of cystinosin. Hum. Mol. Genet. 8:2507-2514. - PubMed
-
- Barak, V., M. Acker, B. Nisman, I. Kalickman, A. Abrahamov, A. Zimran, and S. Yatziv. 1999. Cytokines in Gaucher's disease. Eur. Cytokine Netw. 10:205-210. - PubMed
-
- Bijvoet, A. G., E. H. van de Kamp, M. A. Kroos, J. H. Ding, B. Z. Yang, P. Visser, C. E. Bakker, M. P. Verbeet, B. A. Oostra, A. J. Reuser, and A. T. van der Ploeg. 1998. Generalized glycogen storage and cardiomegaly in a knockout mouse model of Pompe disease. Hum. Mol. Genet. 7:53-62. - PubMed
-
- Broyer, M., M. Guillot, M. C. Gubler, and R. Habib. 1981. Infantile cystinosis: a reappraisal of early and late symptoms. Adv. Nephrol. Necker Hosp. 10:137-166. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
