Generation and characterization of androgen receptor knockout (ARKO) mice: an in vivo model for the study of androgen functions in selective tissues
- PMID: 12370412
- PMCID: PMC129702
- DOI: 10.1073/pnas.212474399
Generation and characterization of androgen receptor knockout (ARKO) mice: an in vivo model for the study of androgen functions in selective tissues
Erratum in
- Proc Natl Acad Sci U S A 2002 Nov 12;99(23):15245. Hung Min-Chi [corrected to Hung Mien-Chie]
Abstract
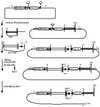
By using a cre-lox conditional knockout strategy, we report here the generation of androgen receptor knockout (ARKO) mice. Phenotype analysis shows that ARKO male mice have a female-like appearance and body weight. Their testes are 80% smaller and serum testosterone concentrations are lower than in wild-type (wt) mice. Spermatogenesis is arrested at pachytene spermatocytes. The number and size of adipocytes are also different between the wt and ARKO mice. Cancellous bone volumes of ARKO male mice are reduced compared with wt littermates. In addition, we found the average number of pups per litter in homologous and heterozygous ARKO female mice is lower than in wt female mice, suggesting potential defects in female fertility and/or ovulation. The cre-lox ARKO mouse provides a much-needed in vivo animal model to study androgen functions in the selective androgen target tissues in female or male mice.
Figures




References
-
- Chang C S, Kokontis J, Liao S T. Science. 1988;240:324–326. - PubMed
-
- Lubahn D B, Joseph D R, Sullivan P M, Willard H F, French F, Wilson E M. Science. 1988;240:327–330. - PubMed
-
- Chang C, Saltzman A, Yeh S, Young W, Keller E, Lee H J, Wang C, Mizokami A. Crit Rev Eukaryotic Gene Expression. 1995;5:97–125. - PubMed
-
- Heinlein C A, Chang C. Endocr Rev. 2002;23:175–200. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

