Mice with chronic norepinephrine deficiency resemble amphetamine-sensitized animals
- PMID: 12370425
- PMCID: PMC129790
- DOI: 10.1073/pnas.212519999
Mice with chronic norepinephrine deficiency resemble amphetamine-sensitized animals
Abstract
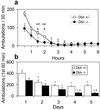
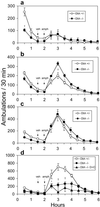
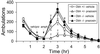
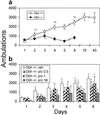
Acute pharmacological blockade of alpha1 adrenoreceptors (ARs) attenuates the locomotor response to amphetamine (LRA). We took a genetic approach to study how norepinephrine (NE) signaling modulates psychostimulant responses by testing LRA in dopamine beta-hydroxylase knockout (Dbh-/-) mice that lack NE. Surprisingly, Dbh-/- animals were hypersensitive to the behavioral effects of amphetamine. Amphetamine (2 mg/kg) elicited greater locomotor activity in Dbh-/- mice compared to controls, whereas 5 mg/kg caused stereotypy in Dbh-/- mice, which is only observed in control mice at higher doses. Prazosin, an alpha1AR antagonist, attenuated LRA in Dbh+/- mice but had no effect in Dbh-/- mice. Changes in the sensitivity of dopamine (DA)-signaling pathways may contribute to the altered amphetamine responses of Dbh-/- mice because they were relatively insensitive to a D1 agonist and hypersensitive to a D2 agonist. Daily amphetamine administration resulted in behavioral sensitization in both Dbh+/- and Dbh-/- mice, demonstrating that NE is not required for the development or expression of behavioral sensitization. Daily prazosin administration blunted but did not completely block locomotor sensitization in Dbh+/- mice, suggesting that alpha1AR signaling contributes to, but is not required for sensitization in Dbh+/- control animals. We conclude that in contrast to acute alpha1AR blockade, chronic NE deficiency induces changes similar to sensitization, perhaps by altering DA-signaling pathways.
Figures


Similar articles
-
Dopamine beta-hydroxylase knockout mice have alterations in dopamine signaling and are hypersensitive to cocaine.Neuropsychopharmacology. 2006 Oct;31(10):2221-30. doi: 10.1038/sj.npp.1301000. Epub 2005 Dec 14. Neuropsychopharmacology. 2006. PMID: 16395294
-
5-HT2A and alpha1b-adrenergic receptors entirely mediate dopamine release, locomotor response and behavioural sensitization to opiates and psychostimulants.Eur J Neurosci. 2004 Dec;20(11):3073-84. doi: 10.1111/j.1460-9568.2004.03805.x. Eur J Neurosci. 2004. PMID: 15579162
-
Effects of SKF-38393, a dopamine D1 receptor agonist on expression of amphetamine-induced behavioral sensitization and expression of immediate early gene arc in prefrontal cortex of rats.Pharmacol Biochem Behav. 2007 May;87(1):56-64. doi: 10.1016/j.pbb.2007.03.020. Epub 2007 Apr 6. Pharmacol Biochem Behav. 2007. PMID: 17499349
-
Toward a molecular understanding of psychostimulant actions using genetically engineered dopamine receptor knockout mice as model systems.J Addict Dis. 2001;20(3):7-18. doi: 10.1300/J069v20n03_02. J Addict Dis. 2001. PMID: 11681595 Review.
-
Norepinephrine deficiency in Parkinson's disease: the case for noradrenergic enhancement.Mov Disord. 2014 Dec;29(14):1710-9. doi: 10.1002/mds.26048. Epub 2014 Oct 9. Mov Disord. 2014. PMID: 25297066 Review.
Cited by
-
α-1 Adrenergic receptors are localized on presynaptic elements in the nucleus accumbens and regulate mesolimbic dopamine transmission.Neuropsychopharmacology. 2012 Aug;37(9):2161-72. doi: 10.1038/npp.2012.68. Epub 2012 May 16. Neuropsychopharmacology. 2012. PMID: 22588352 Free PMC article.
-
Genetic loss of norepinephrine does not alter adult hippocampal neurogenesis in dopamine beta-hydroxylase deficient mice.IBRO Neurosci Rep. 2022 Oct 27;13:420-425. doi: 10.1016/j.ibneur.2022.10.010. eCollection 2022 Dec. IBRO Neurosci Rep. 2022. PMID: 36386600 Free PMC article.
-
Selective D2 and D3 receptor antagonists oppositely modulate cocaine responses in mice via distinct postsynaptic mechanisms in nucleus accumbens.Neuropsychopharmacology. 2019 Jul;44(8):1445-1455. doi: 10.1038/s41386-019-0371-2. Epub 2019 Mar 16. Neuropsychopharmacology. 2019. PMID: 30879021 Free PMC article.
-
Central norepinephrine transmission is required for stress-induced repetitive behavior in two rodent models of obsessive-compulsive disorder.Psychopharmacology (Berl). 2020 Jul;237(7):1973-1987. doi: 10.1007/s00213-020-05512-0. Epub 2020 Apr 20. Psychopharmacology (Berl). 2020. PMID: 32313981 Free PMC article.
-
Norepinephrine is necessary for experience-dependent plasticity in the developing mouse auditory cortex.J Neurosci. 2015 Feb 11;35(6):2432-7. doi: 10.1523/JNEUROSCI.0532-14.2015. J Neurosci. 2015. PMID: 25673838 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

