Uptake of the anticancer drug cisplatin mediated by the copper transporter Ctr1 in yeast and mammals
- PMID: 12370430
- PMCID: PMC137878
- DOI: 10.1073/pnas.162491399
Uptake of the anticancer drug cisplatin mediated by the copper transporter Ctr1 in yeast and mammals
Abstract
Cisplatin is a chemotherapeutic drug used to treat a variety of cancers. Both intrinsic and acquired resistance to cisplatin, as well as toxicity, limit its effectiveness. Molecular mechanisms that underlie cisplatin resistance are poorly understood. Here we demonstrate that deletion of the yeast CTR1 gene, which encodes a high-affinity copper transporter, results in increased cisplatin resistance and reduced intracellular accumulation of cisplatin. Copper, which causes degradation and internalization of Ctr1 protein (Ctr1p), enhances survival of wild-type yeast cells exposed to cisplatin and reduces cellular accumulation of the drug. Cisplatin also causes degradation and delocalization of Ctr1p and interferes with copper uptake in wild-type yeast cells. Mouse cell lines lacking one or both mouse Ctr1 (mCtr1) alleles exhibit increased cisplatin resistance and decreased cisplatin accumulation in parallel with mCtr1 gene dosage. We propose that cisplatin uptake is mediated by the copper transporter Ctr1p in yeast and mammals. The link between Ctr1p and cisplatin transport may explain some cases of cisplatin resistance in humans and suggests ways of modulating sensitivity and toxicity to this important anticancer drug.
Figures

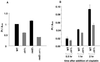

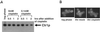

Comment in
-
A copper connection to the uptake of platinum anticancer drugs.Proc Natl Acad Sci U S A. 2002 Oct 29;99(22):13963-5. doi: 10.1073/pnas.232574299. Epub 2002 Oct 21. Proc Natl Acad Sci U S A. 2002. PMID: 12391309 Free PMC article. Review. No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

