Connexin29 expression, immunocytochemistry and freeze-fracture replica immunogold labelling (FRIL) in sciatic nerve
- PMID: 12372015
- PMCID: PMC1803218
- DOI: 10.1046/j.1460-9568.2002.02149.x
Connexin29 expression, immunocytochemistry and freeze-fracture replica immunogold labelling (FRIL) in sciatic nerve
Abstract
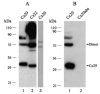
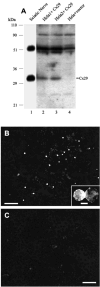
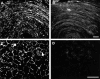
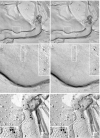
The recently discovered connexin29 (Cx29) was reported to be present in the central and peripheral nervous systems (CNS and PNS), and its mRNA was found in particular abundance in peripheral nerve. The expression and localization of Cx29 protein in sciatic nerve were investigated using an antibody against Cx29. The antibody recognized Cx29 in HeLa cells transfected with Cx29 cDNA, while nontransfected HeLa cells were devoid of Cx29. Immunoblotting of sciatic nerve homogenate revealed monomeric and possibly higher molecular weight forms of Cx29. These were distinguished from connexin32 (Cx32), which also is expressed in peripheral nerve. Double immunofluorescence labelling for Cx29 and Cx32 revealed only partial colocalization of the two connexins, with codistribution at intermittent, conical-shaped striations along nerve fibers. By freeze-fracture replica immunogold labelling (FRIL), Cx32 was found in gap junctions in the outermost layers of myelin, whereas Cx29-immunogold labelling was found only in the innermost layer of myelin in close association with hexagonally arranged intramembrane particle (IMP) 'rosettes' and gap junction-like clusters of IMPs. Although both Cx32 and Cx29 were detected in myelin of normal mice, only Cx29 was present in Schwann cell membranes in Cx32 knockout mice. The results confirm that Cx29 is a second connexin expressed in Schwann cells of sciatic nerve. In addition, Cx29 is present in distinctive IMP arrays in the inner most layer of myelin, adjacent to internodal axonal plasma membranes, where this connexin may have previously unrecognized functions.
Figures





Similar articles
-
Connexin47, connexin29 and connexin32 co-expression in oligodendrocytes and Cx47 association with zonula occludens-1 (ZO-1) in mouse brain.Neuroscience. 2004;126(3):611-30. doi: 10.1016/j.neuroscience.2004.03.063. Neuroscience. 2004. PMID: 15183511 Free PMC article.
-
Role of Connexin-Based Gap Junction Channels in Communication of Myelin Sheath in Schwann Cells.Front Cell Neurosci. 2019 Mar 1;13:69. doi: 10.3389/fncel.2019.00069. eCollection 2019. Front Cell Neurosci. 2019. PMID: 30881289 Free PMC article. Review.
-
Connexin29 is uniquely distributed within myelinating glial cells of the central and peripheral nervous systems.J Neurosci. 2002 Aug 1;22(15):6458-70. doi: 10.1523/JNEUROSCI.22-15-06458.2002. J Neurosci. 2002. PMID: 12151525 Free PMC article.
-
Connexin29 and connexin32 at oligodendrocyte and astrocyte gap junctions and in myelin of the mouse central nervous system.J Comp Neurol. 2003 Sep 22;464(3):356-70. doi: 10.1002/cne.10797. J Comp Neurol. 2003. PMID: 12900929 Free PMC article.
-
Gap junction disorders of myelinating cells.Rev Neurosci. 2010;21(5):397-419. doi: 10.1515/revneuro.2010.21.5.397. Rev Neurosci. 2010. PMID: 21280457 Review.
Cited by
-
Gap junctions couple astrocytes and oligodendrocytes.J Mol Neurosci. 2008 May;35(1):101-16. doi: 10.1007/s12031-007-9027-5. J Mol Neurosci. 2008. PMID: 18236012 Free PMC article. Review.
-
Connexin47, connexin29 and connexin32 co-expression in oligodendrocytes and Cx47 association with zonula occludens-1 (ZO-1) in mouse brain.Neuroscience. 2004;126(3):611-30. doi: 10.1016/j.neuroscience.2004.03.063. Neuroscience. 2004. PMID: 15183511 Free PMC article.
-
Role of Connexin-Based Gap Junction Channels in Communication of Myelin Sheath in Schwann Cells.Front Cell Neurosci. 2019 Mar 1;13:69. doi: 10.3389/fncel.2019.00069. eCollection 2019. Front Cell Neurosci. 2019. PMID: 30881289 Free PMC article. Review.
-
Molecular disruptions of the panglial syncytium block potassium siphoning and axonal saltatory conduction: pertinence to neuromyelitis optica and other demyelinating diseases of the central nervous system.Neuroscience. 2010 Jul 28;168(4):982-1008. doi: 10.1016/j.neuroscience.2009.10.028. Epub 2009 Oct 20. Neuroscience. 2010. PMID: 19850107 Free PMC article. Review.
-
The oligodendroglial precursor cell line Oli-neu represents a cell culture system to examine functional expression of the mouse gap junction gene connexin29 (Cx29).Front Pharmacol. 2013 Jun 28;4:83. doi: 10.3389/fphar.2013.00083. Print 2013. Front Pharmacol. 2013. PMID: 23825458 Free PMC article.
References
-
- Altevogt BM, Paul DL, Goodenough DA. Cloning and characterization of a novel central nervous system specific connexin, mouse connexin29. Mol Biol Cell. 2000;11:1713.
-
- Arroyo EJ, Scherer SS. On the molecular architecture of myelinated fibres. Histochem Cell Biol. 2000;113:1–18. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

