Diffusion-tensor MR imaging of gray and white matter development during normal human brain maturation
- PMID: 12372731
- PMCID: PMC7976805
Diffusion-tensor MR imaging of gray and white matter development during normal human brain maturation
Abstract
Background and purpose: Conventional MR imaging findings of human brain development are thought to result from decreasing water content, increasing macromolecular concentration, and myelination. We use diffusion-tensor MR imaging to test theoretical models that incorporate hypotheses regarding how these maturational processes influence water diffusion in developing gray and white matter.
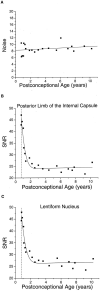
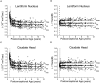
Methods: Experimental data were derived from diffusion-tensor imaging of 167 participants, ages 31 gestational weeks to 11 postnatal years. An isotropic diffusion model was applied to the gray matter of the basal ganglia and thalamus. A model that assumes changes in the magnitude of diffusion while maintaining cylindrically symmetric anisotropy was applied to the white matter of the corpus callosum and internal capsule. Deviations of the diffusion tensor from the ideal model predictions, due to measurement noise, were estimated by using Monte Carlo simulations.
Results: Developing gray matter of the basal ganglia and developing white matter of the internal capsule and corpus callosum largely conformed to theory, with only small departures from model predictions in older children. However, data from the thalamus substantially diverged from predicted values, with progressively larger deviations from the model with increasing participant age.
Conclusion: Changes in water diffusion during maturation of central gray and white matter structures can largely be explained by theoretical models incorporating simple assumptions regarding the influence of brain water content and myelination, although deviations from theory increase as the brain matures. Diffusion-tensor MR imaging is a powerful method for studying the process of brain development, with both scientific and clinical applications.
Figures


References
-
- Barkovich AJ, Kjos BO, Jackson DE Jr, Norman D. Normal brain maturation of the neonatal and infant brain: MR imaging at 1.5 T. Radiology 1988;166:173–180 - PubMed
-
- Basser PJ, Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative diffusion tensor MRI. J Magn Reson B 1996;111:209–219 - PubMed
-
- Conturo TE, McKinstry RC, Akbudak E, Robinson BH. Encoding of anisotropic diffusion with tetrahedral gradients: a general mathematical diffusion formalism and experimental results. Magn Reson Med 1996;35:399–412 - PubMed
-
- Pierpaoli C, Jezzard P, Basser PJ, Barnett A, Di Chiro G. Diffusion tensor MR imaging of the human brain. Radiology 1996;201:637–648 - PubMed
-
- Shimony JS, McKinstry RC, Akbudak E, et al. Quantitative diffusion-tensor anisotropy imaging: normative human data and anatomic analysis. Radiology 1999;212:770–784 - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
