Binding of TGF-beta1 latency-associated peptide (LAP) to alpha(v)beta6 integrin modulates behaviour of squamous carcinoma cells
- PMID: 12373600
- PMCID: PMC2376166
- DOI: 10.1038/sj.bjc.6600545
Binding of TGF-beta1 latency-associated peptide (LAP) to alpha(v)beta6 integrin modulates behaviour of squamous carcinoma cells
Abstract
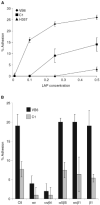
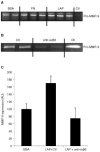
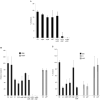
The integrin alpha(v)beta6 is not detectable on normal keratinocytes in vivo but expression is increased significantly in oral squamous cell carcinoma where this heterodimer has been shown to play a role in cell migration, invasion and protease expression. Although regarded initially as a fibronectin receptor, alpha(v)beta6 may bind to arginine-glycine-aspartic acid sequences in other matrix molecules including tenascin and vitronectin. Interestingly, alpha(v)beta6 has also been shown to have high affinity for the TGF-beta1 latency associated peptide and to participate in the activation of the TGF-beta1 latent complex. Since TGF-beta1 is present in squamous carcinomas, it is possible that latency associated peptide may modulate malignant keratinocyte behaviour independently from the classical TGF-beta signalling pathways through its interaction with integrins. We show here that when latency associated peptide is immobilised onto a surface, it acts as an alpha(v)beta6-specific ligand for oral squamous carcinoma cells promoting adhesion and haptotactic migration in addition to alpha(v)beta6-dependent increase in pro-MMP-9 expression. In contrast, even very low concentrations of soluble latency associated peptide (0.1 microg ml(-1)) inhibited alpha(v)beta6-dependent adhesion, migration and invasion. Thus alpha(v)beta6-dependent processes of oral squamous cell carcinoma, is likely to be modulated, not only by the local concentration of latency associated peptide in the stroma, but also whether it is immobilised in the matrix or released as a soluble protein.
Copyright 2002 Cancer Research UK
Figures


Similar articles
-
Role of the alpha(v)beta6 integrin in human oral squamous cell carcinoma growth in vivo and in vitro.Biochem Biophys Res Commun. 2001 Nov 2;288(3):610-8. doi: 10.1006/bbrc.2001.5813. Biochem Biophys Res Commun. 2001. PMID: 11676487
-
Expression of alpha(v)beta6 integrin in oral leukoplakia.Br J Cancer. 2000 Apr;82(8):1433-40. doi: 10.1054/bjoc.1999.1130. Br J Cancer. 2000. PMID: 10780523 Free PMC article.
-
Stromal fibroblasts influence oral squamous-cell carcinoma cell interactions with tenascin-C.Int J Cancer. 1997 Jul 17;72(2):369-76. doi: 10.1002/(sici)1097-0215(19970717)72:2<369::aid-ijc28>3.0.co;2-9. Int J Cancer. 1997. PMID: 9219848
-
Defining the role of integrin alphavbeta6 in cancer.Curr Drug Targets. 2009 Jul;10(7):645-52. doi: 10.2174/138945009788680374. Curr Drug Targets. 2009. PMID: 19601768 Free PMC article. Review.
-
Alphavbeta6 integrin in wound healing and cancer of the oral cavity.J Oral Pathol Med. 2006 Jan;35(1):1-10. doi: 10.1111/j.1600-0714.2005.00374.x. J Oral Pathol Med. 2006. PMID: 16393247 Review.
Cited by
-
Prokaryotic expression, purification and evaluation of anti-cardiac fibrosis activity of recombinant TGF-β latency associated peptide.PeerJ. 2022 Jan 19;10:e12797. doi: 10.7717/peerj.12797. eCollection 2022. PeerJ. 2022. PMID: 35111409 Free PMC article.
-
Engineering a single-chain Fv antibody to alpha v beta 6 integrin using the specificity-determining loop of a foot-and-mouth disease virus.J Mol Biol. 2008 Oct 3;382(2):385-401. doi: 10.1016/j.jmb.2008.07.013. Epub 2008 Jul 16. J Mol Biol. 2008. PMID: 18656482 Free PMC article.
-
Differential regulation of fibronectin expression and fibrillogenesis by autocrine TGF-β1 signaling in malignant and benign mammary epithelial cells.Int J Biochem Cell Biol. 2023 Dec;165:106478. doi: 10.1016/j.biocel.2023.106478. Epub 2023 Oct 21. Int J Biochem Cell Biol. 2023. PMID: 37866655 Free PMC article.
-
Optimized serum stability and specificity of an αvβ6 integrin-binding peptide for tumor targeting.J Biol Chem. 2021 Jan-Jun;296:100657. doi: 10.1016/j.jbc.2021.100657. Epub 2021 Apr 16. J Biol Chem. 2021. PMID: 33857478 Free PMC article.
-
A visual-quantitative analysis of fibroblastic stromagenesis in breast cancer progression.J Mammary Gland Biol Neoplasia. 2004 Oct;9(4):311-24. doi: 10.1007/s10911-004-1403-y. J Mammary Gland Biol Neoplasia. 2004. PMID: 15838602 Review.
References
-
- AhmedNPansinoFClydeRMurthiPQuinnMARiceGEAgrezMVMokSBakerMS2002Overexpression of alphavbeta6 integrin in serous epithelial ovarian cancer regulates extracellular matrix degradation via the plasminogen activation cascade Carcinogenesis 23237244 - PubMed
-
- AkhurstRJBalmainA1999Genetic events and the role of TGF beta in epithelial tumour progression J Pathol 1878290 - PubMed
-
- AkhurstRJDerynckR2001TGF-beta signaling in cancer–a double-edged sword Trends Cell Biol 11S44S51 - PubMed
-
- AnnesJPRifkinDBMungerJS2002The integrin αvβ6 binds and activates latent TGFβ3 FEBS 5116568 - PubMed
-
- ArihiroKKanekoMFujiiSInaiKYokosakiY2000Significance of alpha 9 beta 1 and alpha v beta 6 integrin expression in breast carcinoma Breast Cancer 71926 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

