Ionic regulation of MscK, a mechanosensitive channel from Escherichia coli
- PMID: 12374733
- PMCID: PMC129069
- DOI: 10.1093/emboj/cdf537
Ionic regulation of MscK, a mechanosensitive channel from Escherichia coli
Abstract
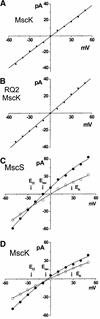
Three gene products that form independent mechanosensitive channel activities have been identified in Escherichia coli. Two of these, MscL and MscS, play a vital role in allowing the cell to survive acute hypotonic stress. Much less is known of the third protein, MscK (KefA). Here, we characterize the MscK channel activity and compare it with the activity of its structural and functional homologue, MscS. While both show a slight anionic preference, MscK appears to be more sensitive to membrane tension. In addition, MscK, but not MscS activity appears to be regulated by external ionic environment, requiring not only membrane tension but also high concentrations of external K(+), NH(4)(+), Rb(+) or Cs(+) to gate; no activity is observed with Na(+), Li(+) or N-methyl-D-glucamine (NMDG). An MscK gain-of-function mutant gates spontaneously in the presence of K(+) or similar ions, and will gate in the presence of Na(+), Li(+) and NMDG, but only when stimulated by membrane tension. Increased sensitivity and the highly regulated nature of MscK suggest a more specialized physiological role than other bacterial mechanosensitive channels.
Figures


References
-
- Berrier C., Besnard,M., Ajouz,B., Coulombe,A. and Ghazi,A. (1996) Multiple mechanosensitive ion channels from Escherichia coli, activated at different thresholds of applied pressure. J. Membr. Biol., 151, 175–187. - PubMed
-
- Blount P. and Moe,P. (1999) Bacterial mechanosensitive channels: integrating physiology, structure and function. Trends Microbiol., 7, 420–424. - PubMed
-
- Blount P., Sukharev,S.I., Moe,P.C., Nagle,S.K. and Kung,C. (1996a) Towards an understanding of the structural and functional properties of MscL, a mechanosensitive channel in bacteria. Biol. Cell, 87, 1–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

